IgG4 Valency Modulates the Pathogenicity of Anti-Neurofascin-155 IgG4 in Autoimmune Nodopathy
- PMID: 35948442
- PMCID: PMC9365386
- DOI: 10.1212/NXI.0000000000200014
IgG4 Valency Modulates the Pathogenicity of Anti-Neurofascin-155 IgG4 in Autoimmune Nodopathy
Abstract
Background and objectives: IgG4 autoantibodies to neurofascin-155 (Nfasc155) are associated with a subgroup of patients with chronic inflammatory demyelinating polyneuropathy (CIDP), currently named autoimmune nodopathy. We previously demonstrated that those antibodies alter conduction along myelinated axons by inducing Nfasc155 depletion and paranode destruction. In blood, IgG4 have the potency to exchange their moiety with other unrelated IgG4 through a process called Fab-arm exchange (FAE). This process results in functionally monovalent antibodies and may affect the pathogenicity of autoantibodies. Here, we examined this issue and whether FAE is beneficial or detrimental for Nfasc155 autoimmune nodopathy.
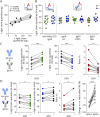
Methods: The bivalency and monospecificity of anti-Nfasc155 were examined by sandwich ELISA in 10 reactive patients, 10 unreactive CIDP patients, and 10 healthy controls. FAE was induced in vitro using reduced glutathione and unreactive IgG4, and the ratio of the κ:λ light chain was monitored. To determine the pathogenic potential of bivalent anti-Nfasc155 IgG4, autoantibodies derived from patients were enzymatically cleaved into monovalent Fab and bivalent F(ab')2 or swapped with unreactive IgG4 and then were injected in neonatal animals.
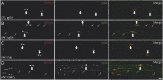
Results: Monospecific bivalent IgG4 against Nfasc155 were detected in the serum of all reactive patients, indicating that a fraction of IgG4 have not undergone FAE in situ. These IgG4 were, nonetheless, capable of engaging into FAE with unreactive IgG4 in vitro, and this decreased the levels of monospecific antibodies and modulated the ratio of the κ:λ light chain. When injected in animals, monovalent anti-Nfasc155 Fab did not alter the formation of paranodes; by contrast, both native anti-Nfasc155 IgG4 and F(ab')2 fragments strongly impaired paranode formation. The promotion of FAE with unreactive IgG4 also strongly diminished the pathogenic potential of anti-Nfasc155 IgG4 in animals and decreased IgG4 clustering on Schwann cells.
Discussion: Our findings demonstrate that monospecific and bivalent anti-Nfasc155 IgG4 are detected in patients and that those autoantibodies are the pathogenic ones. The transformation of anti-Nfasc155 IgG4 into monovalent Fab or functionally monovalent IgG4 through FAE strongly decreases paranodal alterations. Bivalency thus appears crucial for Nfasc155 clustering and paranode destruction.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
