Drinking hydrogen water improves photoreceptor structure and function in retinal degeneration 6 mice
- PMID: 35948585
- PMCID: PMC9365798
- DOI: 10.1038/s41598-022-17903-8
Drinking hydrogen water improves photoreceptor structure and function in retinal degeneration 6 mice
Abstract
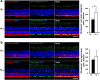
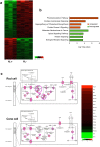
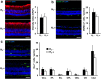
Retinitis pigmentosa (RP) is a genetically heterogeneous group of inherited retinal disorders involving the progressive dysfunction of photoreceptors and the retinal pigment epithelium, for which there is currently no treatment. The rd6 mouse is a natural model of autosomal recessive retinal degeneration. Given the known contributions of oxidative stress caused by reactive oxygen species (ROS) and selective inhibition of potent ROS peroxynitrite and OH·by H2 gas we have previously demonstrated, we hypothesized that ingestion of H2 water may delay the progression of photoreceptor death in rd6 mice. H2 mice showed significantly higher retinal thickness as compared to controls on optical coherence tomography. Histopathological and morphometric analyses revealed higher thickness of the outer nuclear layer for H2 mice than controls, as well as higher counts of opsin red/green-positive cells. RNA sequencing (RNA-seq) analysis of differentially expressed genes in the H2 group versus control group revealed 1996 genes with significantly different expressions. Gene and pathway ontology analysis showed substantial upregulation of genes responsible for phototransduction in H2 mice. Our results show that drinking water high in H2 (1.2-1.6 ppm) had neuroprotective effects and inhibited photoreceptor death in mice, and suggest the potential of H2 for the treatment of RP.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
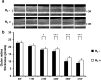
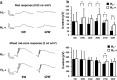
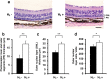
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

