Evidence for a HURP/EB free mixed-nucleotide zone in kinetochore-microtubules
- PMID: 35948594
- PMCID: PMC9365851
- DOI: 10.1038/s41467-022-32421-x
Evidence for a HURP/EB free mixed-nucleotide zone in kinetochore-microtubules
Abstract
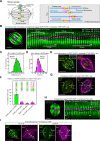
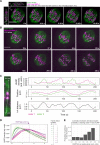
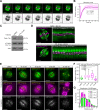
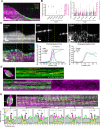
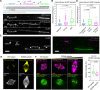
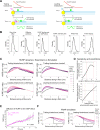
Current models infer that the microtubule-based mitotic spindle is built from GDP-tubulin with small GTP caps at microtubule plus-ends, including those that attach to kinetochores, forming the kinetochore-fibres. Here we reveal that kinetochore-fibres additionally contain a dynamic mixed-nucleotide zone that reaches several microns in length. This zone becomes visible in cells expressing fluorescently labelled end-binding proteins, a known marker for GTP-tubulin, and endogenously-labelled HURP - a protein which we show to preferentially bind the GDP microtubule lattice in vitro and in vivo. We find that in mitotic cells HURP accumulates on the kinetochore-proximal region of depolymerising kinetochore-fibres, whilst avoiding recruitment to nascent polymerising K-fibres, giving rise to a growing "HURP-gap". The absence of end-binding proteins in the HURP-gaps leads us to postulate that they reflect a mixed-nucleotide zone. We generate a minimal quantitative model based on the preferential binding of HURP to GDP-tubulin to show that such a mixed-nucleotide zone is sufficient to recapitulate the observed in vivo dynamics of HURP-gaps.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

