Anti-tumor activity of a T-helper 1 multiantigen vaccine in a murine model of prostate cancer
- PMID: 35948756
- PMCID: PMC9365795
- DOI: 10.1038/s41598-022-17950-1
Anti-tumor activity of a T-helper 1 multiantigen vaccine in a murine model of prostate cancer
Abstract
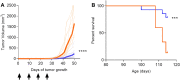
Prostate cancer is one of the few malignancies that includes vaccination as a treatment modality. Elements of an effective cancer vaccine should include the ability to elicit a Type I T-cell response and target multiple antigenic proteins expressed early in the disease. Using existing gene datasets encompassing normal prostate tissue and tumors with Gleason Score ≤ 6 and ≥ 8, 10 genes were identified that were upregulated and conserved in prostate cancer regardless of the aggressiveness of disease. These genes encoded proteins also expressed in prostatic intraepithelial neoplasia. Putative Class II epitopes derived from these proteins were predicted by a combination of algorithms and, using human peripheral blood, epitopes which selectively elicited IFN-γ or IL-10 dominant antigen specific cytokine secretion were determined. Th1 selective epitopes were identified for eight antigens. Epitopes from three antigens elicited Th1 dominant immunity in mice; PSMA, HPN, and AMACR. Each single antigen vaccine demonstrated significant anti-tumor activity inhibiting growth of implanted Myc-Cap cells after immunization as compared to control. Immunization with the combination of antigens, however, was superior to each alone in controlling tumor growth. When vaccination occurred simultaneously to tumor implant, multiantigen immunized mice had significantly smaller tumors than controls (p = 0.002) and a significantly improved overall survival (p = 0.0006). This multiantigen vaccine shows anti-tumor activity in a murine model of prostate cancer.
© 2022. The Author(s).
Conflict of interest statement
M.L.D. is a stockholder in EpiThany and receives grant support from Celgene, EMD Serono, Pfizer, Janssen, and Precigen. M.G., R.B., R.N.D. and M.A.S. are stockholders in Janssen. D.L.C. and M.L.D. are inventors on patents held by the University of Washington. No conflicts are declared for B.C., E.G., A.E.T. or L.C.
Figures


References
-
- Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019;37(13):1051–1061. doi: 10.1200/JCO.18.02031. - DOI - PMC - PubMed
-
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, et al. Pillars article: IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Thl cells. J. Immunol. 1991;146:3444–3451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

