Nebivolol elicits a neuroprotective effect in the cuprizone model of multiple sclerosis in mice: emphasis on M1/M2 polarization and inhibition of NLRP3 inflammasome activation
- PMID: 35948811
- PMCID: PMC9700639
- DOI: 10.1007/s10787-022-01045-4
Nebivolol elicits a neuroprotective effect in the cuprizone model of multiple sclerosis in mice: emphasis on M1/M2 polarization and inhibition of NLRP3 inflammasome activation
Abstract
Background and aim: Multiple sclerosis (MS) is a demyelinating neurodegenerative inflammatory disease affecting mainly young adults. Microgliosis-derived neuroinflammation represents a key hallmark in MS pathology and progression. Nebivolol (Neb) demonstrated antioxidant, anti-inflammatory and neuroprotective properties in several brain pathologies. This study was conducted to investigate the potential neuroprotective effect of Neb in the cuprizone (Cup) model of MS.
Methods: C57Bl/6 mice were fed 0.2% Cup mixed into rodent chow for 5 weeks. Neb (5 and 10 mg/kg/day) was administered by oral gavage during the last 2 weeks.
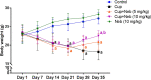
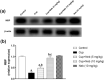
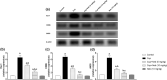
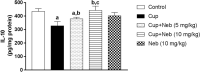
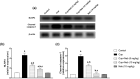
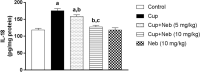
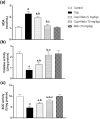
Results: Neb prevented Cup-induced weight loss and motor deficits as evidenced by increased latency to fall in the rotarod test and enhanced locomotor activity as compared to Cup-intoxicated mice. Neb reversed Cup-induced demyelination as confirmed by Luxol fast blue staining and myelin basic protein western blotting. Administration of Neb modulated microglial activation status by suppressing M1 markers (Iba-1, CD86, iNOS, NO and TNF-α) and increasing M2 markers (Arg-1 and IL-10) as compared to Cup-fed mice. Furthermore, Neb hindered NLRP3/caspase-1/IL-18 inflammatory cascade and alleviated oxidative stress by reducing lipid peroxidation, as well as increasing catalase and superoxide dismutase activities.
Conclusion: These findings suggest the potential neuroprotective effect of Neb in the Cup-induced model of MS in mice, at least partially by virtue of shifting microglia towards M2 phenotype, mitigation of NLRP3 inflammasome activation and alleviation of oxidative stress.
Keywords: Cuprizone; M1/M2 polarization; Multiple sclerosis; NLRP3; Nebivolol.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
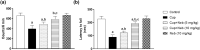

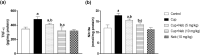

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
