CRISPR/Cas9 genome editing to create nonhuman primate models for studying stem cell therapies for HIV infection
- PMID: 35948929
- PMCID: PMC9363854
- DOI: 10.1186/s12977-022-00604-5
CRISPR/Cas9 genome editing to create nonhuman primate models for studying stem cell therapies for HIV infection
Abstract
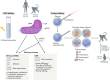
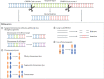
Nonhuman primates (NHPs) are well-established basic and translational research models for human immunodeficiency virus (HIV) infections and pathophysiology, hematopoietic stem cell (HSC) transplantation, and assisted reproductive technologies. Recent advances in CRISPR/Cas9 gene editing technologies present opportunities to refine NHP HIV models for investigating genetic factors that affect HIV replication and designing cellular therapies that exploit genetic barriers to HIV infections, including engineering mutations into CCR5 and conferring resistance to HIV/simian immunodeficiency virus (SIV) infections. In this report, we provide an overview of recent advances and challenges in gene editing NHP embryos and discuss the value of genetically engineered animal models for developing novel stem cell-based therapies for curing HIV.
Keywords: CCR5; CRISPR/Cas9; HIV; HSC transplantation; Nonhuman primates; Pluripotent stem cells; SIV.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Killing two birds with one stone: CRISPR/Cas9 CCR5 knockout hematopoietic stem cells transplantation to treat patients with HIV infection and hematological malignancies concurrently.Clin Exp Med. 2023 Dec;23(8):4163-4175. doi: 10.1007/s10238-023-01129-7. Epub 2023 Jul 27. Clin Exp Med. 2023. PMID: 37500934 Review.
-
CRISPR/Cas9-mediated genome editing in nonhuman primates.Dis Model Mech. 2019 Oct 16;12(10):dmm039982. doi: 10.1242/dmm.039982. Dis Model Mech. 2019. PMID: 31636095 Free PMC article. Review.
-
CRISPR/Cas9-Mediated CCR5 Ablation in Human Hematopoietic Stem/Progenitor Cells Confers HIV-1 Resistance In Vivo.Mol Ther. 2017 Aug 2;25(8):1782-1789. doi: 10.1016/j.ymthe.2017.04.027. Epub 2017 May 17. Mol Ther. 2017. PMID: 28527722 Free PMC article.
-
Generation of SIV-resistant T cells and macrophages from nonhuman primate induced pluripotent stem cells with edited CCR5 locus.Stem Cell Reports. 2022 Apr 12;17(4):953-963. doi: 10.1016/j.stemcr.2022.03.003. Epub 2022 Mar 31. Stem Cell Reports. 2022. PMID: 35364011 Free PMC article.
-
A temperature-sensitive and less immunogenic Sendai virus for efficient gene editing.J Virol. 2024 Dec 17;98(12):e0083224. doi: 10.1128/jvi.00832-24. Epub 2024 Nov 4. J Virol. 2024. PMID: 39494910 Free PMC article.
Cited by
-
Nanomaterials for mRNA-based therapeutics: Challenges and opportunities.Bioeng Transl Med. 2023 Jan 29;8(3):e10492. doi: 10.1002/btm2.10492. eCollection 2023 May. Bioeng Transl Med. 2023. PMID: 37206219 Free PMC article. Review.
-
Whole genome sequencing of CCR5 CRISPR-Cas9-edited Mauritian cynomolgus macaque blastomeres reveals large-scale deletions and off-target edits.Front Genome Ed. 2023 Jan 12;4:1031275. doi: 10.3389/fgeed.2022.1031275. eCollection 2022. Front Genome Ed. 2023. PMID: 36714391 Free PMC article.
-
Antiretrovirals to CCR5 CRISPR/Cas9 gene editing - A paradigm shift chasing an HIV cure.Clin Immunol. 2023 Oct;255:109741. doi: 10.1016/j.clim.2023.109741. Epub 2023 Aug 21. Clin Immunol. 2023. PMID: 37611838 Free PMC article. Review.
-
Killing two birds with one stone: CRISPR/Cas9 CCR5 knockout hematopoietic stem cells transplantation to treat patients with HIV infection and hematological malignancies concurrently.Clin Exp Med. 2023 Dec;23(8):4163-4175. doi: 10.1007/s10238-023-01129-7. Epub 2023 Jul 27. Clin Exp Med. 2023. PMID: 37500934 Review.
-
Atypical initial cleavage patterns minimally impact rhesus macaque in vitro embryo morphokinetics and embryo outgrowth development†.Biol Reprod. 2023 Dec 11;109(6):812-820. doi: 10.1093/biolre/ioad117. Biol Reprod. 2023. PMID: 37688580 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

