Posthemorrhagic hydrocephalus associates with elevated inflammation and CSF hypersecretion via activation of choroidal transporters
- PMID: 35948938
- PMCID: PMC9367104
- DOI: 10.1186/s12987-022-00360-w
Posthemorrhagic hydrocephalus associates with elevated inflammation and CSF hypersecretion via activation of choroidal transporters
Abstract
Introduction: Posthemorrhagic hydrocephalus (PHH) often develops following hemorrhagic events such as intraventricular hemorrhage (IVH) and subarachnoid hemorrhage (SAH). Treatment is limited to surgical diversion of the cerebrospinal fluid (CSF) since no efficient pharmacological therapies are available. This limitation follows from our incomplete knowledge of the molecular mechanisms underlying the ventriculomegaly characteristic of PHH. Here, we aimed to elucidate the molecular coupling between a hemorrhagic event and the subsequent PHH development, and reveal the inflammatory profile of the PHH pathogenesis.
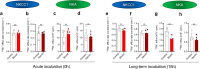
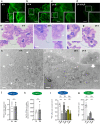
Methods: CSF obtained from patients with SAH was analyzed for inflammatory markers using the proximity extension assay (PEA) technique. We employed an in vivo rat model of IVH to determine ventricular size, brain water content, intracranial pressure, and CSF secretion rate, as well as for transcriptomic analysis. Ex vivo radio-isotope assays of choroid plexus transport were employed to determine the direct effect of choroidal exposure to blood and inflammatory markers, both with acutely isolated choroid plexus and after prolonged exposure obtained with viable choroid plexus kept in tissue culture conditions.
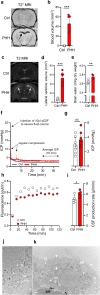
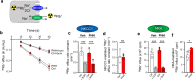
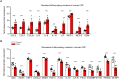
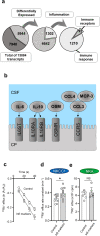
Results: The rat model of IVH demonstrated PHH and associated CSF hypersecretion. The Na+/K+-ATPase activity was enhanced in choroid plexus isolated from IVH rats, but not directly stimulated by blood components. Inflammatory markers that were elevated in SAH patient CSF acted on immune receptors upregulated in IVH rat choroid plexus and caused Na+/K+/2Cl- cotransporter 1 (NKCC1) hyperactivity in ex vivo experimental conditions.
Conclusions: CSF hypersecretion may contribute to PHH development, likely due to hyperactivity of choroid plexus transporters. The hemorrhage-induced inflammation detected in CSF and in the choroid plexus tissue may represent the underlying pathology. Therapeutic targeting of such pathways may be employed in future treatment strategies towards PHH patients.
Keywords: Biomarkers; Cerebrospinal fluid; Choroid plexus; Immune response; Intraventricular hemorrhage; Posthemorrhagic hydrocephalus; Subarachnoid hemorrhage.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have not competing interests.
Figures
Similar articles
-
Lysophosphatidic acid as a CSF lipid in posthemorrhagic hydrocephalus that drives CSF accumulation via TRPV4-induced hyperactivation of NKCC1.Fluids Barriers CNS. 2022 Sep 6;19(1):69. doi: 10.1186/s12987-022-00361-9. Fluids Barriers CNS. 2022. PMID: 36068581 Free PMC article.
-
Elevated CSF inflammatory markers in patients with idiopathic normal pressure hydrocephalus do not promote NKCC1 hyperactivity in rat choroid plexus.Fluids Barriers CNS. 2021 Dec 4;18(1):54. doi: 10.1186/s12987-021-00289-6. Fluids Barriers CNS. 2021. PMID: 34863228 Free PMC article.
-
NLRP3 inflammasome-mediated choroid plexus hypersecretion contributes to hydrocephalus after intraventricular hemorrhage via phosphorylated NKCC1 channels.J Neuroinflammation. 2022 Jun 21;19(1):163. doi: 10.1186/s12974-022-02530-x. J Neuroinflammation. 2022. PMID: 35729645 Free PMC article.
-
Cerebrospinal fluid hypersecretion in pediatric hydrocephalus.Neurosurg Focus. 2016 Nov;41(5):E10. doi: 10.3171/2016.8.FOCUS16278. Neurosurg Focus. 2016. PMID: 27798982 Review.
-
Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: Established mechanisms and proposed pathways.J Neurosci Res. 2020 Jan;98(1):105-120. doi: 10.1002/jnr.24394. Epub 2019 Feb 21. J Neurosci Res. 2020. PMID: 30793349 Free PMC article. Review.
Cited by
-
Morphometric Study of the Initial Ventricular Indices to Predict the Complications and Outcome of Aneurysmal Subarachnoid Hemorrhage.J Clin Med. 2023 Mar 29;12(7):2585. doi: 10.3390/jcm12072585. J Clin Med. 2023. PMID: 37048667 Free PMC article.
-
Posthemorrhagic Hydrocephalus in Patients with Subarachnoid Hemorrhage Occurs Independently of CSF Osmolality.Int J Mol Sci. 2023 Jul 14;24(14):11476. doi: 10.3390/ijms241411476. Int J Mol Sci. 2023. PMID: 37511234 Free PMC article.
-
Cranial Ultrasound in the Management of Hydrocephalus in Newborns: A Case Series.Children (Basel). 2025 Mar 27;12(4):419. doi: 10.3390/children12040419. Children (Basel). 2025. PMID: 40310071 Free PMC article.
-
Novel insights into non-coding RNAs and their role in hydrocephalus.Neural Regen Res. 2026 Feb 1;21(2):636-647. doi: 10.4103/NRR.NRR-D-24-00963. Epub 2024 Dec 16. Neural Regen Res. 2026. PMID: 39688559 Free PMC article.
-
Outcomes of the 2019 hydrocephalus association workshop, "Driving common pathways: extending insights from posthemorrhagic hydrocephalus".Fluids Barriers CNS. 2023 Jan 13;20(1):4. doi: 10.1186/s12987-023-00406-7. Fluids Barriers CNS. 2023. PMID: 36639792 Free PMC article. Review.
References
-
- Mazzola C, Choudhri A, Auguste K, Limbrick D, Rogido M, Mitchell L, Flannery A. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr. 2014;14(Suppl 1):8–23. doi: 10.3171/2014.7.PEDS14322. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

