Characterizing the patient experience during neoadjuvant therapy for pancreatic ductal adenocarcinoma: A qualitative study
- PMID: 35949220
- PMCID: PMC9244990
- DOI: 10.4251/wjgo.v14.i6.1175
Characterizing the patient experience during neoadjuvant therapy for pancreatic ductal adenocarcinoma: A qualitative study
Abstract
Background: Neoadjuvant therapy (NT) has increasingly been utilized for patients with localized pancreatic ductal adenocarcinoma (PDAC). It is the recommended approach for borderline resectable (BR) and locally advanced (LA) cancers and an increasingly utilized option for potentially resectable (PR) disease. Despite its increased use, little research has focused on patient-centered metrics among patients undergoing NT, including patient experiences, preferences, and recommendations. A better understanding of all aspects of the patient experience during NT may identify opportunities to design interventions aimed at improving quality of life; it may also facilitate the completion of NT and receipt of surgery, ultimately optimizing long-term outcomes.
Aim: To understand the experience of patients initiating and receiving NT to identify opportunities to improve neoadjuvant cancer care delivery.
Methods: Semi-structured interviews of patients with localized PDAC during NT were conducted to explore their experience initiating and receiving NT. Interviews took place between August 2020 and October 2021. Due to the descriptive nature of the research, questions were open ended. Interviews were conducted over the phone, audio recorded and then transcribed. All interviews were coded by two independent researchers using NVivo 12, iteratively identifying themes until thematic saturation was achieved. An integrative approach to qualitative analysis was used, utilizing both inductive and deductive methods.
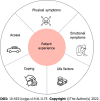
Results: A total of 12 patients with localized PDAC were interviewed. Patients with BR (n = 7), PR (n = 2), and LA (n = 3) cancers participated in the study. All patients indicated that choosing NT was the doctor's recommendation, while most reported not being familiar with the concept of NT (n = 11) and that NT was presented as the only option (n = 8). Five themes describing the patient experience emerged: physical symptoms, emotional symptoms, coping mechanisms, access to care, and life factors. The most commonly cited recommendation for improving the experience of NT was improved education before and during NT (n = 7). Patients highlighted the need for more information on the rationale behind choosing NT prior to surgery, the anticipated surgery and its likelihood of surgery occurring after NT, as well as general information prior to starting NT treatment. The need for seeing different members of the healthcare team, including ancillary services was also frequently cited as a recommendation for improving the experience of NT (n = 5).
Conclusion: This study provides a framework to allow for a better understanding of the PDAC patient experience during NT and highlights opportunities to improve quality and quantity of life outcomes.
Keywords: Neoadjuvant therapy; Pancreatic ductal adenocarcinoma; Patient experience; Patient-centered care; Qualitative research; Quality of life.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There are no conflicts of interest to report.
Figures
Similar articles
-
Multi-specialty physician perspectives on barriers and facilitators to the use of neoadjuvant therapy for pancreatic ductal adenocarcinoma.HPB (Oxford). 2022 Jun;24(6):833-840. doi: 10.1016/j.hpb.2021.10.010. Epub 2021 Oct 25. HPB (Oxford). 2022. PMID: 34764009 Free PMC article.
-
Evaluating the caregiver experience during neoadjuvant therapy for pancreatic ductal adenocarcinoma.J Surg Oncol. 2024 Mar;129(4):775-784. doi: 10.1002/jso.27558. Epub 2023 Dec 8. J Surg Oncol. 2024. PMID: 38063046
-
Neoadjuvant therapy for localized pancreatic ductal adenocarcinoma.Minerva Surg. 2024 Jun;79(3):315-325. doi: 10.23736/S2724-5691.23.10150-X. Epub 2024 Feb 22. Minerva Surg. 2024. PMID: 38385797 Review.
-
Patient Preferences for Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma.Pancreas. 2022 Jul 1;51(6):657-662. doi: 10.1097/MPA.0000000000002083. Epub 2022 Sep 13. Pancreas. 2022. PMID: 36099500
-
Patient experience and quality of life during neoadjuvant therapy for pancreatic cancer: a systematic review and study protocol.Support Care Cancer. 2021 Jun;29(6):3009-3016. doi: 10.1007/s00520-020-05813-2. Epub 2020 Oct 8. Support Care Cancer. 2021. PMID: 33030596
Cited by
-
From Surviving to Living (on): A Grounded Theory Study on Coping in People with Pancreatic Cancer.J Patient Exp. 2023 Nov 20;10:23743735231215605. doi: 10.1177/23743735231215605. eCollection 2023. J Patient Exp. 2023. PMID: 38148769 Free PMC article.
-
Patient Perceptions of Care Coordination during Neoadjuvant Therapy for Gastrointestinal Cancers: A Mixed Methods Analysis.J Gastrointest Cancer. 2024 Jun;55(2):862-868. doi: 10.1007/s12029-024-01030-w. Epub 2024 Feb 14. J Gastrointest Cancer. 2024. PMID: 38351391 Free PMC article.
-
Best Practices for Delivering Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma.JAMA Surg. 2025 Feb 1;160(2):172-180. doi: 10.1001/jamasurg.2024.5191. JAMA Surg. 2025. PMID: 39630427
-
Supportive Care Needs and Related Interventions in Patients with Pancreatic Cancer and Their Informal Caregivers: A Scoping Review.J Gastrointest Cancer. 2025 Apr 16;56(1):98. doi: 10.1007/s12029-025-01218-8. J Gastrointest Cancer. 2025. PMID: 40234313 Free PMC article.
-
The Association Between Patient-Reported Outcomes and Surgical Attrition During Neoadjuvant Therapy for Gastrointestinal Malignancies.J Gastrointest Cancer. 2024 Dec 11;56(1):31. doi: 10.1007/s12029-024-01153-0. J Gastrointest Cancer. 2024. PMID: 39661262
References
-
- Cloyd JM, Shen C, Santry H, Bridges J, Dillhoff M, Ejaz A, Pawlik TM, Tsung A. Disparities in the Use of Neoadjuvant Therapy for Resectable Pancreatic Ductal Adenocarcinoma. J Natl Compr Canc Netw. 2020;18:556–563. - PubMed
-
- de Geus SW, Eskander MF, Bliss LA, Kasumova GG, Ng SC, Callery MP, Tseng JF. Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis. Surgery. 2017;161:592–601. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials