Substitution mutational signatures in whole-genome-sequenced cancers in the UK population
- PMID: 35949260
- PMCID: PMC7613262
- DOI: 10.1126/science.abl9283
Substitution mutational signatures in whole-genome-sequenced cancers in the UK population
Abstract
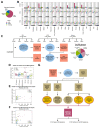
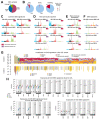
Whole-genome sequencing (WGS) permits comprehensive cancer genome analyses, revealing mutational signatures, imprints of DNA damage and repair processes that have arisen in each patient's cancer. We performed mutational signature analyses on 12,222 WGS tumor-normal matched pairs, from patients recruited via the UK National Health Service. We contrasted our results to two independent cancer WGS datasets, the International Cancer Genome Consortium (ICGC) and Hartwig Foundation, involving 18,640 WGS cancers in total. Our analyses add 40 single and 18 double substitution signatures to the current mutational signature tally. Critically, we show for each organ, that cancers have a limited number of 'common' signatures and a long tail of 'rare' signatures. We provide a practical solution for utilizing this concept of common versus rare signatures in future analyses.
Conflict of interest statement
Competing interests: AD, XZ, HRD and SNZ hold patents or have submitted applications on clinical algorithms of mutational signatures (MMRDetect (number pending), HRDetect: PCT/EP2017/060294, Clinical use of signatures: PCT/EP2017/060289, Rearrangement sigantures methods: PCT/EP2017/060279, Clinical predictor: PCT/EP2017/060298, Hotspots for chromosomal rearrangements: PCT/EP2017/060298) and during this project, served advisory roles for AstraZeneca, Artios Pharma and the Scottish Genomes Project.
Figures





References
-
- Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

