Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial)
- PMID: 35949295
- PMCID: PMC9218617
- DOI: 10.1159/000521665
Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial)
Abstract
Introduction: It remains unclear which surgery or radiofrequency ablation (RFA) is the more effective treatment for small hepatocellular carcinoma (HCC). We aimed to compare survival between patients undergoing surgery (surgery group) and patients undergoing RFA (RFA group).
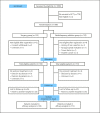
Methods: We conducted a randomized controlled trial involving 49 institutions in Japan. Patients with Child-Pugh scores ≤7, largest HCC diameter ≤3 cm, and ≤3 HCC nodules were considered eligible. The co-primary endpoints were recurrence-free survival (RFS) and overall survival (OS). The current study reports the final result of RFS, and the follow-up of OS is still ongoing.
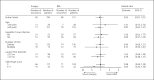
Results: During 2009-2015, 308 patients were registered. After excluding ineligible patients, the surgery and RFA groups included 150 and 151 patients, respectively. Baseline factors did not differ significantly between the groups. In both groups, 90% of patients had solitary HCC. The median largest HCC diameter was 1.8 cm (interquartile range [IQR], 1.5-2.2 cm) in the surgery group and 1.8 cm (IQR, 1.5-2.3 cm) in the RFA group. The median procedure duration (274 vs. 40 min, p < 0.01) and the median duration of hospital stay (17 days vs. 10 days, p < 0.01) were longer in the surgery group than in the RFA group. RFS did not differ significantly between the groups as the median RFS was 3.5 (95% confidence interval [CI], 2.6-5.1) years in the surgery group and 3.0 (95% CI, 2.4-5.6) years in the RFA group (hazard ratio, 0.92; 95% CI, 0.67-1.25; p = 0.58).
Discussion/conclusion: Our study did not show which surgery or RFA is the better treatment option for small HCC.
Keywords: Ablation; Hepatectomy; Hepatocellular carcinoma; Liver resection; Randomized controlled trial.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
Kiyoshi Hasegawa: lecture fees from Taiho, Bayer Yakuhin, MSD K.K., and Eisai; research funding from Chugai, Otsuka, Bristol-Myers Squibb, Takeda, Taiho, Yakult, Eisai, AbbVie, Bayer Yakuhin, Kowa, Shimazu, and Nipro. Masatoshi Kudo: lecture fees from Eisai, Bayer, MDS, BMS, EA Pharma, Eli Lilly, and Chugai; research funding from Eisai, Takeda, Otsuka, Taiho, EA Pharma, Gilead Sciences, AbbVie, Sumitomo Dainippon Pharma, Chugai, and Ono Pharma; advisory role with Eisai, Ono, MSD, BMS, and Roche. Mitsuo Shimada: research funding from AbbVie, Astellas, Bayer Yakuhin, Chugai, Covidien, EA Pharma, Eisai, Novartis Pharma, Ono, Taiho, and Takeda. Yoshikuni Kawaguchi: lecture fees from Olympus, CONMED, and Otsuka. Ryosuke Tateishi: lecture fees from Chugai, Daiichi Sankyo, Eisai, GE Health Care, and Gilead; advisory roles with AstraZeneca and Shionogi, Medtronic, MSD, Otsuka, Sumitomo Dainihon Pharma, Kowa Company, Takeda, Ono, and Shionogi. Kazuhiko Koike: research funding from MSD, AbbVie, Gilead Sciences, Otsuka, ASKA Pharma, EA Pharma, Dainippon-Sumitomo, Chugai, Eli Lilly, Taiho, Yakult, Bayer, Takeda, Tanabe-Mitsubishi, Myaln EPD, Taisho, Rohto, Eisai, and Zeria. Masatoshi Kudo is the editor-in-chief of Liver Cancer. Namiki Izumi and Norihiro Kokudo are associate editors of Liver Cancer. Kiyoshi Hasegawa is an editorial board member of Liver Cancer. None of the other authors have potential conflicts of interest to declare.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. - PubMed
-
- Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998 Nov;28((5)):1241–6. - PubMed
-
- European Association for the Study of the Liver Electronic address eee, European association for the study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69((1)):182–236. - PubMed
-
- Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan society of hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019 Jul 23;49((10)):1109. - PubMed
-
- Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993 Jul;9((4)):298–304. - PubMed
LinkOut - more resources
Full Text Sources