Cigarette smoke extract stimulates human pulmonary artery smooth muscle cell proliferation: Role of inflammation and oxidative stress
- PMID: 35949310
- PMCID: PMC9320202
- DOI: 10.22038/IJBMS.2022.64170.14133
Cigarette smoke extract stimulates human pulmonary artery smooth muscle cell proliferation: Role of inflammation and oxidative stress
Abstract
Objectives: Cigarette smoke may play a direct role in proliferation of human pulmonary artery smooth muscle cells (HPASMCs). However, the mechanism involved and the effect of interventions remain unclear. We aimed to evaluate the effect of cigarette smoke extract (CSE) on HPASMCs, explore the role of inflammation and oxidative stress, and the effects of Tempol and PDTC in this process.
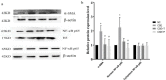
Materials and methods: HPASMCs were subjected to normal control (NC), CSE, CSE+Tempol (CSE+T), and CSE+PDTC (CSE+P) groups. Proliferation of HPASMCs was measured by CCK-8 and Western blot. TNF-α, IL-6, MDA, and SOD levels were determined by ELISA and commercial kits. Nuclear translocation of NF-κB p65 was evaluated by western blot.
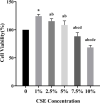
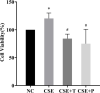
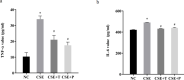
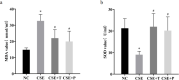
Results: 1%, 2.5%, and 5% CSE all promoted proliferation of HPASMCs, and effect of 1% CSE was the most significant, however, 7.5% and 10% CSE inhibited viability of cells (all P<0.05). Compared with the NC group, TNF-α, IL-6, and MDA levels increased, SOD activity decreased (all P<0.05), and NF-κB p65 expression in nuclei increased (P=0.04) in the CSE group. Tempol and PDTC inhibited the proliferation of HPASMCs induced by CSE (all P<0.05). And compared with the CSE group, TNF-α, IL-6, and MDA levels in CSE+T and CSE+P groups decreased, while SOD activity increased (all P<0.05). Tempol reduced the expression of NF-κB p65 in nuclei but did not achieve a significant difference (P=0.08). PDTC inhibited the nuclear translocation of NF-κB p65 (P=0.03).
Conclusion: CSE stimulates HPASMCs proliferation in a certain concentration range. The CSE-induced proliferation of HPASMCs involved excessive inflammatory response and oxidative stress. Tempol and PDTC attenuate these effects of CSE on HPASMCs.
Keywords: Cigarette smoking; Inflammation; NF-κB; Oxidative stress; Pulmonary arterial - hypertension.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures
Similar articles
-
Involvement of TRPC1 and Cyclin D1 in Human Pulmonary Artery Smooth Muscle Cells Proliferation Induced by Cigarette Smoke Extract.Curr Med Sci. 2020 Dec;40(6):1085-1091. doi: 10.1007/s11596-020-2290-1. Epub 2021 Jan 11. Curr Med Sci. 2020. PMID: 33428136
-
[Cyclin D1 is involved in human pulmonary artery smooth muscle cells proliferation and migration induced by cigarette smoke extract].Sheng Li Xue Bao. 2010 Apr 25;62(2):156-62. Sheng Li Xue Bao. 2010. PMID: 20401451 Chinese.
-
Cigarette smoke extract promotes human pulmonary artery smooth muscle cells proliferation through protein kinase C alpha-dependent induction of cyclin D1.Chin Med J (Engl). 2010 Dec;123(24):3663-70. Chin Med J (Engl). 2010. PMID: 22166648
-
Effect of nuclear factor-kappa B on vascular endothelial growth factor mRNA expression of human pulmonary artery smooth muscle cells in hypoxia.J Huazhong Univ Sci Technolog Med Sci. 2004;24(1):9-12, 18. doi: 10.1007/BF02830694. J Huazhong Univ Sci Technolog Med Sci. 2004. PMID: 15165104
-
Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models.Pulm Pharmacol Ther. 2010 Oct;23(5):411-9. doi: 10.1016/j.pupt.2010.05.004. Epub 2010 May 23. Pulm Pharmacol Ther. 2010. PMID: 20566376
Cited by
-
Bilirubin regulates cell death type by alleviating macrophage mitochondrial dysfunction caused by cigarette smoke extract.Redox Rep. 2024 Dec;29(1):2382946. doi: 10.1080/13510002.2024.2382946. Epub 2024 Jul 29. Redox Rep. 2024. PMID: 39074442 Free PMC article.
-
Smooth Muscle Cell Phenotypic Switch Induced by Traditional Cigarette Smoke Condensate: A Holistic Overview.Int J Mol Sci. 2023 Mar 29;24(7):6431. doi: 10.3390/ijms24076431. Int J Mol Sci. 2023. PMID: 37047404 Free PMC article.
-
Gypenosides alleviates MCT-induced pulmonary arterial hypertension in rats by targeting oxidative stress, inflammation, and apoptosis.Iran J Basic Med Sci. 2025;28(8):1019-1026. doi: 10.22038/ijbms.2025.82437.17822. Iran J Basic Med Sci. 2025. PMID: 40584450 Free PMC article.
-
Determination of pulmonary vessel alteration in Chinese male smokers by quantitative computed tomography measurements: a retrospective study.Quant Imaging Med Surg. 2024 May 1;14(5):3289-3301. doi: 10.21037/qims-23-1758. Epub 2024 Apr 12. Quant Imaging Med Surg. 2024. PMID: 38720846 Free PMC article.
-
Pathological mechanisms of cigarette smoking, dietary, and sedentary lifestyle risks in vascular dysfunction: mitochondria as a common target of risk factors.Pflugers Arch. 2023 Jul;475(7):857-866. doi: 10.1007/s00424-023-02806-y. Epub 2023 Mar 30. Pflugers Arch. 2023. PMID: 36995495 Free PMC article. Review.
References
-
- Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166:314–322. - PubMed
-
- Thabut G, Dauriat G, Stern JB, Logeart D, Lévy A, Marrash-Chahla R, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. - PubMed
LinkOut - more resources
Full Text Sources
