Comparison of a histone deacetylase inhibitor plus exemestane with exemestane alone in hormone receptor-positive advanced breast cancer that progressed on prior endocrine therapy: A meta-analysis
- PMID: 35949321
- PMCID: PMC9353490
- DOI: 10.3892/etm.2022.11512
Comparison of a histone deacetylase inhibitor plus exemestane with exemestane alone in hormone receptor-positive advanced breast cancer that progressed on prior endocrine therapy: A meta-analysis
Abstract
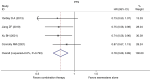
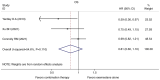
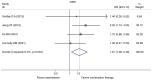
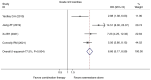
Currently, endocrine therapy is the standard treatment for hormone receptor-positive advanced breast cancer (ABC). Despite the high sensitivity of anti-estrogen therapy, many breast cancer patients still experience disease progression, relapse, and reduced overall survival (OS) because of endocrine resistance. Several underlying mechanisms of this phenomenon include a change in hormone receptor expression, mutations in ESR1 and modification of important signaling pathways, but thus far none of these can be defined as the complete explanation. Additionally, it has been shown that in some breast cancers, expression of the estrogen receptor (ER) can be repressed by epigenetic modifications such as DNA methylation and histone deacetylation, and this could be a mechanism for endocrine resistance. Interestingly, although the efficacy of the combination of histone deacetylase (HADC) inhibitors and exemestane in hormone receptor-positive ABC that progressed on prior endocrine therapy has been investigated in several studies, whether pharmacologic blocking of HDAC activity acts as a therapeutic strategy remains highly controversial. Herein, we conducted a meta-analysis to evaluate the efficacy and safety of an HDAC inhibitor plus exemestane vs. exemestane alone in this setting. Our meta-analysis demonstrated that the combination group exhibited significantly prolonged progression-free survival (PFS) [hazard ratio (HR)=0.776, 95% confidence interval (CI)=0.675-0.892, P=0.000] and an improved objective response rate (ORR) (RR=1.612, 95% CI=1.085-2.396, P=0.018) compared to those treated with exemestane alone. Additionally, in terms of OS, the combination group failed to achieve a significant clinical OS benefit (HR=0.811, 95% CI=0.596-1.104, P=0.183). Although grade 3/4 toxicities were more common in the combination group, those toxicities were mostly asymptomatic and manageable. In conclusion, the addition of an HDAC inhibitor to exemestane significantly improves PFS over exemestane alone in hormone receptor-positive ABC patients who progressed on previous endocrine therapy. Identification of novel biomarkers to select patients who will benefit from this combination strategy is a high priority.
Keywords: breast cancer; combination therapy; endocrine resistance; histone deacetylase inhibitors; metastasis.
Copyright: © Xu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
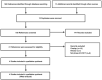
Figures
References
-
- El Sayed R, El Jamal L, El Iskandarani S, Kort J, Abdel Salam M, Assi H. Endocrine and targeted therapy for hormone-receptor-positive, HER2-Negative advanced breast cancer: Insights to sequencing treatment and overcoming resistance based on clinical trials. Front Oncol. 2019;9(510) doi: 10.3389/fonc.2019.00510. - DOI - PMC - PubMed
-
- Baselga J, Im SA, Iwata H, Cortés J, De Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–916. doi: 10.1016/S1470-2045(17)30376-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
