Adipose-derived stem cells postpone the progression of Sjögren's syndrome by upregulating the Hippo signaling pathway
- PMID: 35949326
- PMCID: PMC9353405
- DOI: 10.3892/etm.2022.11524
Adipose-derived stem cells postpone the progression of Sjögren's syndrome by upregulating the Hippo signaling pathway
Abstract
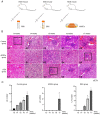
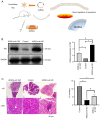
The aim of the present study was to explore the effect and mechanism of action of adipose-derived stem cells (ADSCs) on Sjögren syndrome (SS) to develop novel and more effective methods for SS treatment. ADSCs, dexamethasone or normal saline was injected into the submandibular gland (SMG) of three 12-week-old non-obese diabetic (NOD) mice. The degree of lymphocyte infiltration was considered as a criterion for judging disease progression, hematoxylin and eosin staining was performed to observe the pathological state, and the expression levels of TAZ, E-cadherin and α-catenin were assessed by western blotting. ADSC transplantation triggered an inhibitory effect on the progression of SS, which was slightly stronger compared with that of dexamethasone treatment. This was found to be related to the Hippo signaling pathway. In addition, TAZ protein expression levels decreased gradually with the progression of the disease; immunofluorescence staining showed that the expression of E-cadherin and TAZ followed similar trends. Notably, the expression of TAZ, p-TAZ, E-cadherin and α-catenin in NOD mice were lower compared with that in Control mice. Similarly, the ratio of p-TAZ/TAZ also decreased, which means that the activation level of Hippo signal pathway decreased. The results suggest that ADSCs may exert a therapeutic effect against SS and may postpone its progression by upregulating the Hippo signaling pathway.
Keywords: Hippo signaling pathway; Sjögren's syndrome; adipose-derived stem cell.
Copyright: © Li et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The Hippo signaling pathway is required for salivary gland development and its dysregulation is associated with Sjogren's syndrome.Lab Invest. 2013 Nov;93(11):1203-18. doi: 10.1038/labinvest.2013.114. Epub 2013 Sep 30. Lab Invest. 2013. PMID: 24080911 Free PMC article.
-
Mechanism of cAMP-PKA Signaling Pathway Mediated by Shaoyao Gancao Decoction () on Regulation of Aquaporin 5 and Muscarinic Receptor 3 Levels in Sjögren's Syndrome.Chin J Integr Med. 2020 Jul;26(7):502-509. doi: 10.1007/s11655-020-3205-5. Epub 2020 Jul 6. Chin J Integr Med. 2020. PMID: 32632716
-
Pharmacological activation of TAZ enhances osteogenic differentiation and bone formation of adipose-derived stem cells.Stem Cell Res Ther. 2018 Mar 7;9(1):53. doi: 10.1186/s13287-018-0799-z. Stem Cell Res Ther. 2018. PMID: 29514703 Free PMC article.
-
Wnt, RSPO and Hippo Signalling in the Intestine and Intestinal Stem Cells.Genes (Basel). 2018 Jan 8;9(1):20. doi: 10.3390/genes9010020. Genes (Basel). 2018. PMID: 29316729 Free PMC article. Review.
-
Targeting the Hippo Signaling Pathway for Tissue Regeneration and Cancer Therapy.Genes (Basel). 2016 Aug 30;7(9):55. doi: 10.3390/genes7090055. Genes (Basel). 2016. PMID: 27589805 Free PMC article. Review.
Cited by
-
Research progress on Hippo signaling pathway effector molecules in rheumatic immune system diseases.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jun 19;53(3):376-381. doi: 10.3724/zdxbyxb-2023-0567. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38899353 Free PMC article. Review. Chinese, English.
-
Effect of adipose tissue-derived stem cells therapy on clinical response in patients with primary Sjogren's syndrome.Sci Rep. 2023 Aug 19;13(1):13521. doi: 10.1038/s41598-023-40802-5. Sci Rep. 2023. PMID: 37598237 Free PMC article. Clinical Trial.
-
Lycorine upregulates the expression of RMB10, promotes apoptosis and inhibits the proliferation and migration of cervical cancer cells.Int J Mol Med. 2022 Dec;50(6):145. doi: 10.3892/ijmm.2022.5201. Epub 2022 Nov 11. Int J Mol Med. 2022. PMID: 36367172 Free PMC article.
References
-
- Fasano S, Mauro D, Macaluso F, Xiao F, Zhao Y, Lu L, Guggino G, Ciccia F. Pathogenesis of primary Sjögren's syndrome beyond B lymphocytes. Clin Exp Rheumatol. 2020;38 (Suppl 126):S315–S323. - PubMed
LinkOut - more resources
Full Text Sources
