Resveratrol ameliorates oxaliplatin-induced neuropathic pain via anti-inflammatory effects in rats
- PMID: 35949346
- PMCID: PMC9353538
- DOI: 10.3892/etm.2022.11523
Resveratrol ameliorates oxaliplatin-induced neuropathic pain via anti-inflammatory effects in rats
Abstract
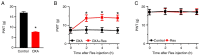
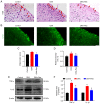
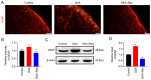
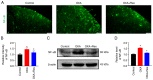
Oxaliplatin (OXA) is a common chemotherapy drug and exhibits clinical activity in several cancer types. Its anticancer clinical effect is frequently accompanied by neurotoxicity. The symptoms include paresthesia and pain, which adversely affect the quality of life of patients. In the present study, five consecutive intraperitoneal injections of 4 mg/kg OXA were used to mimic chemotherapy in rats. OXA administration induced mechanical allodynia, activated spinal astrocytes and triggered the inflammatory response. To explore potential therapeutic options for OXA-induced neuropathic pain, resveratrol (Res) was intrathecally injected into the spinal cord of OXA-treated rats. Paw withdrawal threshold values of OXA-treated rats were increased, indicating an antinociception effect of Res on OXA-induced pain. Additionally, Res treatment reduced the levels of glial fibrillary acidic protein, TNF-α, IL-1β and NF-κB, which were upregulated in OXA-treated rats (compared with control). Furthermore, Auto Dock data showed that Res binds to cyclooxygenase-2 (COX-2) through six hydrogen bonds. Western blot analysis and reactive oxygen species (ROS) assays indicated that Res treatment decreased COX-2 expression and suppressed ROS production. In summary, intrathecal injection of Res reduced the spinal COX-2-mediated ROS generation and inflammatory reaction, suppressed astrocytic activation, and alleviated OXA-induced neuropathic pain.
Keywords: cyclooxygenase-2; oxaliplatin-induced neuropathic pain; resveratrol; spinal inflammation.
Copyright: © Dong et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Emodin suppresses oxaliplatin-induced neuropathic pain by inhibiting COX2/NF-κB mediated spinal inflammation.J Biochem Mol Toxicol. 2023 Jan;37(1):e23229. doi: 10.1002/jbt.23229. Epub 2022 Oct 2. J Biochem Mol Toxicol. 2023. PMID: 36184831
-
2-Bromopalmitate decreases spinal inflammation and attenuates oxaliplatin-induced neuropathic pain via reducing Drp1-mediated mitochondrial dysfunction.PLoS One. 2022 Oct 31;17(10):e0275428. doi: 10.1371/journal.pone.0275428. eCollection 2022. PLoS One. 2022. PMID: 36315519 Free PMC article.
-
Curcumin relieves oxaliplatin-induced neuropathic pain via reducing inflammation and activating antioxidant response.Cell Biol Int. 2024 Jun;48(6):872-882. doi: 10.1002/cbin.12153. Epub 2024 Mar 13. Cell Biol Int. 2024. PMID: 38480956
-
Anti-allodynic effect of Buja in a rat model of oxaliplatin-induced peripheral neuropathy via spinal astrocytes and pro-inflammatory cytokines suppression.BMC Complement Altern Med. 2017 Jan 14;17(1):48. doi: 10.1186/s12906-017-1556-z. BMC Complement Altern Med. 2017. PMID: 28088201 Free PMC article.
-
Anti-allodynic and anti-inflammatory effects of 17α-hydroxyprogesterone caproate in oxaliplatin-induced peripheral neuropathy.J Peripher Nerv Syst. 2019 Mar;24(1):100-110. doi: 10.1111/jns.12307. Epub 2019 Feb 25. J Peripher Nerv Syst. 2019. PMID: 30680838
Cited by
-
Resveratrol suppresses neuroinflammation to alleviate mechanical allodynia by inhibiting Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway in a rat model of spinal cord injury.Front Mol Neurosci. 2023 Feb 16;16:1116679. doi: 10.3389/fnmol.2023.1116679. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36873101 Free PMC article.
-
Xanthohumol relieves arthritis pain in mice by suppressing mitochondrial-mediated inflammation.Mol Pain. 2023 Jan-Dec;19:17448069231204051. doi: 10.1177/17448069231204051. Mol Pain. 2023. PMID: 37699859 Free PMC article.
-
GSK-3β inhibition alleviates arthritis pain via reducing spinal mitochondrial reactive oxygen species level and inflammation.PLoS One. 2023 Apr 14;18(4):e0284332. doi: 10.1371/journal.pone.0284332. eCollection 2023. PLoS One. 2023. PMID: 37058473 Free PMC article.
-
Enzyme-Instructed Aggregation/Dispersion of Fluorophores for Near-Infrared Fluorescence Imaging In Vivo.Molecules. 2023 Jul 12;28(14):5360. doi: 10.3390/molecules28145360. Molecules. 2023. PMID: 37513233 Free PMC article. Review.
-
Intraganglionic reactive oxygen species mediate inflammatory pain and hyperalgesia through TRPA1 in the rat.Front Pain Res (Lausanne). 2023 May 30;4:1204057. doi: 10.3389/fpain.2023.1204057. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37325677 Free PMC article.
References
-
- Yi Y, Li L, Song F, Li P, Chen M, Ni S, Zhang H, Zhou H, Zeng S, Jiang H. L-tetrahydropalmatine reduces oxaliplatin accumulation in the dorsal root ganglion and mitochondria through selectively inhibiting the transporter-mediated uptake thereby attenuates peripheral neurotoxicity. Toxicology. 2021;459(152853) doi: 10.1016/j.tox.2021.152853. - DOI - PubMed
-
- Takeshita E, Ishibashi K, Koda K, Oda N, Yoshimatsu K, Sato Y, Oya M, Yamaguchi S, Nakajima H, Momma T, et al. The updated five-year overall survival and long-term oxaliplatin-related neurotoxicity assessment of the FACOS study. Surg Today. 2021;51:1309–1319. doi: 10.1007/s00595-021-02230-8. - DOI - PubMed
-
- Furgała-Wojas A, Kowalska M, Nowaczyk A, Fijałkowski Ł, Sałat K. Comparison of bromhexine and its active metabolite-ambroxol as potential analgesics reducing oxaliplatin-induced neuropathic pain-pharmacodynamic and molecular docking studies. Curr Drug Metab. 2020;21:548–561. doi: 10.2174/1389200221666200711155632. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
