Diagnostic Test Accuracy of Urine C-peptide Creatinine Ratio for the Correct Identification of the Type of Diabetes: A Systematic Review
- PMID: 35949364
- PMCID: PMC9354948
- DOI: 10.17925/EE.2022.18.1.2
Diagnostic Test Accuracy of Urine C-peptide Creatinine Ratio for the Correct Identification of the Type of Diabetes: A Systematic Review
Abstract
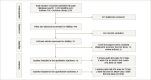
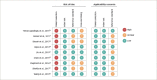
Objective: To examine the accuracy of urine c-peptide creatinine ratio (UCPCR) for identifying the type of diabetes in appropriate clinical settings. Design: Systematic review of test accuracy studies on patients with different forms of diabetes. Data sources: Medline, Embase and Cochrane library databases from 1 January 2000 to 15 November 2020. Eligibility criteria: Studies reporting the use of UCPCR for diagnosing patients with type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM) and monogenic forms of diabetes (categorized as maturity-onset diabetes of the young [MODY]). Study selection and data synthesis: Two reviewers independently assessed articles for inclusion and assessed the methodological quality of the studies using the Quality Assessment of Diagnostic Accuracy Studies-2 tool, with input from a third reviewer to reach consensus when there was a dispute. Meta-analysis was performed with the studies reporting complete data to derive the pooled sensitivity, specificity and diagnostic odds ratio (DOR), and narrative synthesis only for those with incomplete data. Results: Nine studies with 4,488 patients were included in the qualitative synthesis, while only four of these (915 patients) had complete data and were included in the quantitative synthesis. All the studies had moderate risk of bias and applicability concerns. Meta-analysis of three studies (n=130) revealed sensitivity, specificity and DOR of 84.4% (95% confidence interval [CI] 68.1-93.2%), 91.6% (82.8-96.1%) and 59.9 (32.8-106.0), respectively, for diagnosing T1DM using a UCPCR cut-off of <0.2 nmol/mmol. For participants with T2DM (three studies; n=739), UCPCR >0.2 nmol/mmol was associated with sensitivity, specificity and DOR of 92.8% (84.2-96.9%), 81.6% (61.3-92.5%) and 56.9 (31.3-103.5), respectively. For patients with MODY in the appropriate clinical setting, a UCPCR cut-off of >0.2 nmol/mmol showed sensitivity, specificity and DOR of 85.2% (73.1-92.4%), 98.0% (92.4-99.5%) and 281.8 (57.5-1,379.7), respectively. Conclusions: Based on studies with moderate risk of bias and applicability concerns, UCPCR confers moderate to high sensitivity, specificity, and DOR for correctly identifying T1DM, T2DM and monogenic diabetes in appropriate clinical settings. Large multinational studies with multi-ethnic participation among different age groups are necessary before this test can be routinely used in clinical practice. Study registration: Protocol was registered as PROSPERO CRD42017060633.
Keywords: C-peptide; endogenous insulin reserve; maturity-onset diabetes of the young (MODY); monogenic diabetes; type 1 diabetes mellitus (T1DM); type 2 diabetes mellitus (T2DM); urine C-peptide creatinine ratio (UCPCR).
© Touch Medical Media 2022.
Conflict of interest statement
Disclosures: Joseph M Pappachan, Bhuvana Sunil, Cornelius J Fernandez, Ian M Lahart and Ambika P Ashraf have no financial or non-financial relationships or activities to declare in relation to this article.
Figures




Similar articles
-
Clinical Implications of Urinary C-Peptide Creatinine Ratio in Patients with Different Types of Diabetes.J Diabetes Res. 2019 Aug 7;2019:1747684. doi: 10.1155/2019/1747684. eCollection 2019. J Diabetes Res. 2019. PMID: 31485449 Free PMC article.
-
Urinary C-peptide creatinine ratio to differentiate type 2 diabetes mellitus from type 1 in pediatric patients.Eur J Pediatr. 2020 Jul;179(7):1115-1120. doi: 10.1007/s00431-020-03606-7. Epub 2020 Feb 13. Eur J Pediatr. 2020. PMID: 32052124 Clinical Trial.
-
Urinary C-Peptide/Creatinine Ratio Can Distinguish Maturity-Onset Diabetes of the Young from Type 1 Diabetes in Children and Adolescents: A Single-Center Experience.Horm Res Paediatr. 2015;84(1):54-61. doi: 10.1159/000375410. Epub 2015 Mar 17. Horm Res Paediatr. 2015. PMID: 25792383
-
What Markers Best Guide the Timing of Reimplantation in Two-stage Exchange Arthroplasty for PJI? A Systematic Review and Meta-analysis.Clin Orthop Relat Res. 2018 Oct;476(10):1972-1983. doi: 10.1097/01.blo.0000534680.87622.43. Clin Orthop Relat Res. 2018. PMID: 30794241 Free PMC article.
-
Diagnostic Accuracy of Neutrophil Gelatinase-Associated Lipocalin for Predicting Early Diabetic Nephropathy in Patients with Type 1 and Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis.J Appl Lab Med. 2019 Jul;4(1):78-94. doi: 10.1373/jalm.2018.028530. Epub 2019 Mar 1. J Appl Lab Med. 2019. PMID: 31639710
Cited by
-
Rising tide: The global surge of type 2 diabetes in children and adolescents demands action now.World J Diabetes. 2024 May 15;15(5):797-809. doi: 10.4239/wjd.v15.i5.797. World J Diabetes. 2024. PMID: 38766426 Free PMC article.
-
Urinary C-Peptide to Creatinine Ratio (UCPCR) as Indicator for Metabolic Risk in Apparently Healthy Adults-A BioPersMed Cohort Study.Nutrients. 2023 Apr 25;15(9):2073. doi: 10.3390/nu15092073. Nutrients. 2023. PMID: 37432211 Free PMC article.
References
-
- Matthews DR, Rudenski AS, Burnett MA. et al. The half-life of endogenous insulin and C-peptide in man assessed by somatostatin suppression. Clin Endocrinol (Oxf). 1985;23:71–9. - PubMed
-
- Meistas MT, Rendell M, Margolis S, Kowarski AA. Estimation of the secretion rate of insulin from the urinary excretion rate of C-peptide. Study in obese and diabetic subjects. Diabetes. 1982;31:449–53. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
