Care Navigation Increases Initiation of Hepatitis C Treatment After Release From Prison in a Prospective Randomized Controlled Trial: The C-LINK Study
- PMID: 35949401
- PMCID: PMC9356682
- DOI: 10.1093/ofid/ofac350
Care Navigation Increases Initiation of Hepatitis C Treatment After Release From Prison in a Prospective Randomized Controlled Trial: The C-LINK Study
Abstract
Background: Prison-based hepatitis C treatment is safe and effective; however, many individuals are released untreated due to time or resource constraints. On community re-entry, individuals face a number of immediate competing priorities, and in this context, linkage to hepatitis C care is low. Interventions targeted at improving healthcare continuity after prison release have yielded positive outcomes for other health diagnoses; however, data regarding hepatitis C transitional care are limited.
Methods: We conducted a prospective randomized controlled trial comparing a hepatitis C care navigator intervention with standard of care for individuals released from prison with untreated hepatitis C infection. The primary outcome was prescription of hepatitis C direct-acting antivirals (DAA) within 6 months of release.
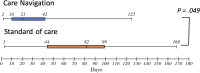
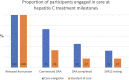
Results: Forty-six participants were randomized. The median age was 36 years and 59% were male. Ninety percent (n = 36 of 40) had injected drugs within 6 months before incarceration. Twenty-two were randomized to care navigation and 24 were randomized to standard of care. Individuals randomized to the intervention were more likely to commence hepatitis C DAAs within 6 months of release (73%, n = 16 of 22 vs 33% n = 8 of 24, P < .01), and the median time between re-entry and DAA prescription was significantly shorter (21 days [interquartile range {IQR}, 11-42] vs 82 days [IQR, 44-99], P = .049).
Conclusions: Care navigation increased hepatitis C treatment uptake among untreated individuals released from prison. Public policy should support similar models of care to promote treatment in this high-risk population. Such an approach will help achieve hepatitis C elimination as a public health threat.
Keywords: DAA; elimination; hepatitis C; prison; transitional care.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. D. reports investigator-initiated research funding to institution from Gilead and AbbVie and honoraria to institution from Gilead. M. H. reports investigator-initiated research funding from Gilead Sciences and AbbVie. D. I. reports honoraria for speaking duties from AbbVie. A. J. T. reports the following: involvement in advisory boards for AbbVie, Gilead Sciences, Roche Diagnostics, BMS, Merck, Immunocore, Janssen, Assembly Biosciences, Arbutus, Vir Biotechnology, Eisai, Ipsen, and Bayer; honoraria for speaking duties from AbbVie, Gilead Sciences, Roche, and BMS; and investigator-initiated research funding from Gilead Sciences, BMS, AbbVie, and Roche Molecular Systems. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- World Health Organization . Combating hepatitis B and C to reach elimination by 2030: advocacy brief. Available: https://apps.who.int/iris/handle/10665/206453. Accessed 3 July 2020.
-
- Scott N, McBryde ES, Thompson A, Doyle JS, Hellard ME. Treatment scale-up to achieve global HCV incidence and mortality elimination targets: a cost-effectiveness model. Gut 2017; 66:1507–15. - PubMed
-
- Papaluca T, McDonald L, Craigie A, et al. Outcomes of treatment for hepatitis C in prisoners using a nurse-led, statewide model of care. J Hepatol 2019; 70:839–46. - PubMed
-
- Palmer A, Papaluca T, Stoové M, et al. A costing analysis of a state-wide, nurse-led hepatitis C treatment model in prison. Int J Drug Policy 2021; 94:103203. - PubMed

