Does Routine Follow-Up after Patients Have Completed Adjuvant Therapy for Early-Stage Breast Cancer at a Cancer Center Improve Prognosis?
- PMID: 35949420
- PMCID: PMC9247563
- DOI: 10.1159/000519533
Does Routine Follow-Up after Patients Have Completed Adjuvant Therapy for Early-Stage Breast Cancer at a Cancer Center Improve Prognosis?
Abstract
Introduction: This study aimed to assess whether follow-up of patients with operative breast cancer at cancer centres (CCs) improved prognosis compared with follow-up by family physicians (FPs).
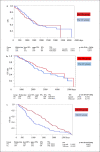
Methods: The study included 254 patients who relapsed within 7 years from the first postoperative period. The patients were divided into two groups according to the follow-up facility: the CC and FP groups (the follow-up of patients was structured in the same way between FPs and CCs). There are 146 and 108 cases of recurrence in the CC and FP groups, respectively. The analysis targets of the two groups were determined using the propensity matching method based on the following 7 factors: oestrogen receptor status, progesterone receptor status, human epidermal growth factor receptor 2 status, St. Gallen category, menopausal status, surgical procedure, and receipt of postoperative chemotherapy at the time of surgery. Overall survival (OS) in both groups was analysed using the Kaplan-Meier method and compared using the log-rank test.
Results: Overall, 97 patients each in the CC and FP groups who relapsed were analysed using the propensity matching method. The median recurrence-free survival periods were 1,676 and 994 days in the FP and CC groups, respectively, and were significantly longer in the FP group. However, the median OS starting from the day of surgery was 3,424 and 2,794 days in the FP and CC groups, respectively, with no significant difference.
Conclusion: This study revealed that regular follow-up at CCs did not improve survival compared with regular follow-up by FPs.
Keywords: Breast cancer; Family physician; Routine follow-up.
Copyright © 2021 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care.J Clin Oncol. 2006 Feb 20;24(6):848-55. doi: 10.1200/JCO.2005.03.2235. Epub 2006 Jan 17. J Clin Oncol. 2006. PMID: 16418496 Clinical Trial.
-
Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Oncol. 2016 Mar;17(3):367-377. doi: 10.1016/S1470-2045(15)00551-3. Epub 2016 Feb 10. Lancet Oncol. 2016. PMID: 26874901 Clinical Trial.
-
[A multi-center retrospective study of perioperative chemotherapy for gastric cancer based on real-world data].Zhonghua Wei Chang Wai Ke Za Zhi. 2021 May 25;24(5):403-412. doi: 10.3760/cma.j.cn.441530-20200111-00014. Zhonghua Wei Chang Wai Ke Za Zhi. 2021. PMID: 34000769 Chinese.
-
Adjuvant chemotherapy in early breast cancer.Dan Med J. 2016 May;63(5):B5222. Dan Med J. 2016. PMID: 27127018 Review.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
References
-
- Smith T, Davidson N, Schapira D; American Society of Clinical Oncology. 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999 Mar;17((3)):1080–2. - PubMed
-
- Grunfeld E, Noorani H, McGahan L, Paszat L, Coyle D, van Walraven C, et al. Surveillance mammography after treatment of primary breast cancer: a systematic review. Breast. 2002 Jun;11((3)):228–35. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

