Nicotinic receptors modulate antitumor therapy response in triple negative breast cancer cells
- PMID: 35949430
- PMCID: PMC9244968
- DOI: 10.5306/wjco.v13.i6.505
Nicotinic receptors modulate antitumor therapy response in triple negative breast cancer cells
Abstract
Background: Triple negative breast cancer is more aggressive than other breast cancer subtypes and constitutes a public health problem worldwide since it has high morbidity and mortality due to the lack of defined therapeutic targets. Resistance to chemotherapy complicates the course of patients' treatment. Several authors have highlighted the participation of nicotinic acetylcholine receptors (nAChR) in the modulation of conventional chemotherapy treatment in cancers of the airways. However, in breast cancer, less is known about the effect of nAChR activation by nicotine on chemotherapy treatment in smoking patients.
Aim: To investigate the effect of nicotine on paclitaxel treatment and the signaling pathways involved in human breast MDA-MB-231 tumor cells.
Methods: Cells were treated with paclitaxel alone or in combination with nicotine, administered for one or three 48-h cycles. The effect of the addition of nicotine (at a concentration similar to that found in passive smokers' blood) on the treatment with paclitaxel (at a therapeutic concentration) was determined using the 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The signaling mediators involved in this effect were determined using selective inhibitors. We also investigated nAChR expression, and ATP "binding cassette" G2 drug transporter (ABCG2) expression and its modulation by the different treatments with Western blot. The effect of the treatments on apoptosis induction was determined by flow cytometry using annexin-V and 7AAD markers.
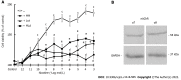
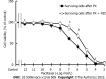
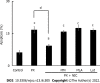
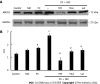
Results: Our results confirmed that treatment with paclitaxel reduced MDA-MB-231 cell viability in a concentration-dependent manner and that the presence of nicotine reversed the cytotoxic effect induced by paclitaxel by involving the expression of functional α7 and α9 nAChRs in these cells. The action of nicotine on paclitaxel treatment was linked to modulation of the protein kinase C, mitogen-activated protein kinase, extracellular signal-regulated kinase, and NF-κB signaling pathways, and to an up-regulation of ABCG2 protein expression. We also detected that nicotine significantly reduced the increase in cell apoptosis induced by paclitaxel treatment. Moreover, the presence of nicotine reduced the efficacy of paclitaxel treatment administered in three cycles to MDA-MB-231 tumor cells.
Conclusion: Our findings point to nAChRs as responsible for the decrease in the chemotherapeutic effect of paclitaxel in triple negative tumors. Thus, nAChRs should be considered as targets in smoking patients.
Keywords: Breast cancer; Drug therapy; Drug transporter; Nicotinic acetylcholine receptors; Paclitaxel; Signal transduction.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors certify that they have no conflicts of interest (including but not limited to commercial, personal, political, intellectual or religious interests) for this article.
Figures



References
-
- Paumgartten FJR, Gomes-Carneiro MR, Oliveira ACAX. The impact of tobacco additives on cigarette smoke toxicity: a critical appraisal of tobacco industry studies. Cad Saude Publica. 2017;33 Suppl 3:e00132415. - PubMed
-
- DiSilvio B, Baqdunes M, Alhajhusain A, Cheema T. Smoking Addiction and Strategies for Cessation. Crit Care Nurs Q. 2021;44:33–48. - PubMed
-
- Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

