Immunomodulatory components of Trichinella spiralis excretory-secretory products with lactose-binding specificity
- PMID: 35949491
- PMCID: PMC9360477
- DOI: 10.17179/excli2022-4954
Immunomodulatory components of Trichinella spiralis excretory-secretory products with lactose-binding specificity
Abstract
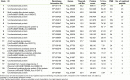
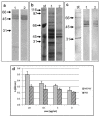
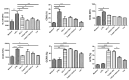
The immunomodulatory potential of Trichinella spiralis muscle larvae excretory-secretory products (ES L1) has been well documented in vitro on dendritic cells (DCs) and in animal models of autoimmune diseases. ES L1 products possess the potential to induce tolerogenic DCs and consequently trigger regulatory mechanisms that maintain immune homeostasis. The use of ES L1 as a potential treatment for various inflammatory disorders proved to be beneficial in animal models, although the precise immunomodulatory factors have not yet been identified. This study aimed at the isolation and characterization of ES L1 components that possess galectin family member properties. Galectin-1-like proteins (TsGal-1-like) were isolated from ES L1 based on the assumption of the existence of a lactose-specific carbohydrate-recognition domain and were recognized by anti-galectin-1 antibodies in Western blot. This TsGal-1-like isolate, similar to galectin-1, induced DCs with tolerogenic properties and hence, the capacity to polarize T cell response towards a regulatory type. This was reflected by a significantly increased percentage of CD4+CD25+Foxp3+ regulatory T cells and significantly increased expression of IL-10 and TGF-β within this cell population. Proteomic analysis of TsGal-1-like isolate by mass spectrometry identified nineteen proteins, seven with annotated function after blast analysis against a database for T. spiralis and the UniProt database. To our surprise, none of the identified proteins possesses homology with known galectin family members. Nevertheless, the isolated components of ES L1 possess certain galectin-1 properties, such as specific lactose binding and the potential to elicit a regulatory immune response, so it would be worth further investigating the structure of sugar binding within isolated proteins and its biological significance.
Keywords: Trichinella spiralis; galectin-1; immunomodulation; proteomics.
Copyright © 2022 Ilic et al.
Figures

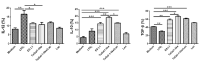


References
-
- Ahmad N, Gabius HJ, Sabesan S, Oscarson S, Brewer CF. Thermodynamic binding studies of bivalent oligosaccharides to galectin-1, galectin-3, and the carbohydrate recognition domain of galectin-3. Glycobiology. 2004;14:817–25. - PubMed
-
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. - PubMed
-
- Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–1357. - PubMed
-
- Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem. 1995;270:5207–5212. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
