Phenological segregation suggests speciation by time in the planktonic diatom Pseudo-nitzschia allochrona sp. nov
- PMID: 35949533
- PMCID: PMC9352866
- DOI: 10.1002/ece3.9155
Phenological segregation suggests speciation by time in the planktonic diatom Pseudo-nitzschia allochrona sp. nov
Abstract
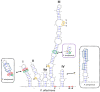
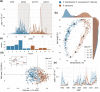
The processes leading to the emergence of new species are poorly understood in marine plankton, where weak physical barriers and homogeneous environmental conditions limit spatial and ecological segregation. Here, we combine molecular and ecological information from a long-term time series and propose Pseudo-nitzschia allochrona, a new cryptic planktonic diatom, as a possible case of speciation by temporal segregation. The new species differs in several genetic markers (18S, 28S and ITS rDNA fragments and rbcL) from its closest relatives, which are morphologically very similar or identical, and is reproductively isolated from its sibling species P. arenysensis. Data from a long-term plankton time series show P. allochrona invariably occurring in summer-autumn in the Gulf of Naples, where its closely related species P. arenysensis, P. delicatissima, and P. dolorosa are instead found in winter-spring. Temperature and nutrients are the main factors associated with the occurrence of P. allochrona, which could have evolved in sympatry by switching its phenology and occupying a new ecological niche. This case of possible speciation by time shows the relevance of combining ecological time series with molecular information to shed light on the eco-evolutionary dynamics of marine microorganisms.
Keywords: cryptic species; diatoms; eco‐evolutionary dynamics; long‐term ecological research (LTER); phenology.
© 2022 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
We declare no conflict of interest.
Figures



References
-
- Ajani, P. , Murray, S. , Hallegraeff, G. , Lundholm, N. , Gillings, M. , Brett, S. , & Armand, L. (2013). The diatom genus Pseudo‐nitzschia (Bacillariophyceae) in New South Wales, Australia: Morphotaxonomy, molecular phylogeny, toxicity, and distribution. Journal of Phycology, 49(4), 765–785. 10.1111/jpy.12087 - DOI - PubMed
-
- Almandoz, G. O. , Ferreyra, G. A. , Schloss, I. R. , Dogliotti, A. I. , Rupolo, V. , Paparazzo, F. E. , Esteves, J. L. , & Ferrario, M. E. (2008). Distribution and ecology of Pseudo‐nitzschia species (Bacillariophyceae) in surface waters of the Weddell Sea (Antarctica). Polar Biology, 31, 429–442.
-
- Amato, A. , Orsini, L. , D'Alelio, D. , & Montresor, M. (2005). Life cycle, size reduction patterns, and ultrastructure of the pennate planktonic diatom Pseudo‐nitzschia delicatissima (Bacillariophyceae). Journal of Phycology, 41, 542–556.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

