Connexin 43 overexpression induces lung cancer angiogenesis in vitro following phosphorylation at Ser279 in its C-terminus
- PMID: 35949588
- PMCID: PMC9353244
- DOI: 10.3892/ol.2022.13413
Connexin 43 overexpression induces lung cancer angiogenesis in vitro following phosphorylation at Ser279 in its C-terminus
Abstract
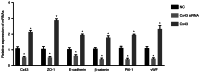
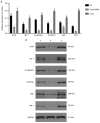
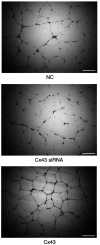
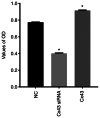
Blocking angiogenesis can inhibit tumor growth and metastasis. However, the mechanism underlying regulation of lung cancer angiogenesis remains unclear. The gap junction protein connexin 43 (Cx43) is implicated in angiogenesis. The aim of the present study was to determine the role of Cx43 in angiogenesis in vitro and its signaling pathways. Human pulmonary microvascular endothelial cells were transfected with Cx43-targeting siRNA or Cx43-overexpressing recombinant plasmid vector. Reverse transcription-quantitative polymerase chain reaction and western blotting were performed to determine Cx43, zonula occludens-1 (ZO-1), E-cadherin, β-catenin, von Willebrand factor (vWF), and plasminogen activator inhibitor-1 (PAI-1) mRNA and protein expression levels, respectively. Tyr265, Ser279, Ser368, and Ser373 phosphorylation levels in the C-terminus of Cx43 and intracellular and membranal Cx43 contents were determined using western blotting. Additionally, immunofluorescence, tube formation, Cell Counting Kit-8, and Transwell migration assays were performed. The results revealed that compared with that in the control samples, Cx43, ZO-1, E-cadherin, β-catenin, vWF, and PAI-1 mRNA and protein expression were significantly increased in the Cx43 overexpression group and significantly decreased in the Cx43-knockdown group. Moreover, the phosphorylation level of Ser279 as well as cell proliferation and migration rates were markedly increased in the Cx43 overexpression group, and tube formation revealed that the potential of angiogenesis was also increased. Conversely, in the Cx43-knockdown group, the phosphorylation level of Ser279 and cell proliferation and migration rates were reduced, and the potential of angiogenesis was greatly impaired. Under Cx43 overexpression, membranal Cx43 content was significantly increased, whereas under Cx43 knockdown, it was significantly reduced. Therefore, Cx43 overexpression could induce pulmonary angiogenesis in vitro by promoting cell proliferation and migration and activating ZO-1, E-cadherin, β-catenin, vWF, and PAI-1. This may be achieved by promoting phosphorylation and activation of the intracellular signal site Ser279 at the C-terminus of Cx43.
Keywords: C terminus; angiogenesis; connexin 43; human pulmonary microvascular endothelial cells; phosphorylation activation.
Copyright: © Zhou et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








Similar articles
-
The connexin 43/ZO-1 complex regulates cerebral endothelial F-actin architecture and migration.Am J Physiol Cell Physiol. 2015 Nov 1;309(9):C600-7. doi: 10.1152/ajpcell.00155.2015. Epub 2015 Aug 19. Am J Physiol Cell Physiol. 2015. PMID: 26289751 Free PMC article.
-
Relationship of Cx43 regulation of vascular permeability to osteopontin-tight junction protein pathway after sepsis in rats.Am J Physiol Regul Integr Comp Physiol. 2018 Jan 1;314(1):R1-R11. doi: 10.1152/ajpregu.00443.2016. Epub 2017 Oct 4. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 28978514
-
PKCɛ mediates serine phosphorylation of connexin43 induced by lysophosphatidylcholine in neonatal rat cardiomyocytes.Toxicology. 2013 Dec 6;314(1):11-21. doi: 10.1016/j.tox.2013.08.001. Epub 2013 Aug 20. Toxicology. 2013. PMID: 23973256
-
Interaction of α Carboxyl Terminus 1 Peptide With the Connexin 43 Carboxyl Terminus Preserves Left Ventricular Function After Ischemia-Reperfusion Injury.J Am Heart Assoc. 2019 Aug 20;8(16):e012385. doi: 10.1161/JAHA.119.012385. Epub 2019 Aug 19. J Am Heart Assoc. 2019. PMID: 31422747 Free PMC article.
-
Specific Cx43 phosphorylation events regulate gap junction turnover in vivo.FEBS Lett. 2014 Apr 17;588(8):1423-9. doi: 10.1016/j.febslet.2014.01.049. Epub 2014 Feb 4. FEBS Lett. 2014. PMID: 24508467 Free PMC article. Review.
Cited by
-
Connexin-43 in Cancer: Above and Beyond Gap Junctions!Cancers (Basel). 2024 Dec 16;16(24):4191. doi: 10.3390/cancers16244191. Cancers (Basel). 2024. PMID: 39766090 Free PMC article. Review.
-
The roles of connexins and gap junctions in the progression of cancer.Cell Commun Signal. 2023 Jan 13;21(1):8. doi: 10.1186/s12964-022-01009-9. Cell Commun Signal. 2023. PMID: 36639804 Free PMC article. Review.
-
Mutations of the Cx43 Gene in Non-Small Cell Lung Cancer: Association with Aberrant Localization of Cx43 Protein Expression and Tumor Progression.Medicina (Kaunas). 2024 Oct 7;60(10):1641. doi: 10.3390/medicina60101641. Medicina (Kaunas). 2024. PMID: 39459427 Free PMC article.
-
Bibliometric analysis and visualization of Connexin 43 in the field of solid tumor research(2000-2024).Front Immunol. 2025 May 9;16:1588828. doi: 10.3389/fimmu.2025.1588828. eCollection 2025. Front Immunol. 2025. PMID: 40416989 Free PMC article.
-
Gap Junctional Interaction of Endothelial Progenitor Cells (EPC) with Endothelial Cells Induces Angiogenic Network Formation In Vitro.Int J Mol Sci. 2025 May 18;26(10):4827. doi: 10.3390/ijms26104827. Int J Mol Sci. 2025. PMID: 40429968 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
