Propofol inhibits the malignant development of osteosarcoma U2OS cells via AMPK/FΟΧO1-mediated autophagy
- PMID: 35949604
- PMCID: PMC9353775
- DOI: 10.3892/ol.2022.13430
Propofol inhibits the malignant development of osteosarcoma U2OS cells via AMPK/FΟΧO1-mediated autophagy
Abstract
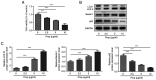
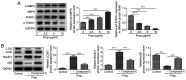
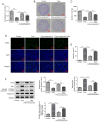
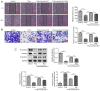
It has previously been reported that propofol regulates the development of human osteosarcoma (OS). However, the specific molecular mechanisms underlying the effect of propofol on OS remain poorly understood. Therefore, the aim of the present study was to explore the effects of propofol on OS U2OS cells and the potential underlying mechanism. The Cell Counting Kit-8 and colony formation assays were performed to assess cell viability and proliferation. Furthermore, cell apoptosis was assessed using the TUNEL assay and western blotting. Wound healing and Transwell assays were performed to evaluate OS cell migration and invasion abilities, respectively. The protein expression levels of epithelial-mesenchymal transition (EMT)-, autophagy- and adenosine monophosphate-activated protein kinase (AMPK)/FOXO1 signaling pathway-related proteins were also determined using western blotting. The results demonstrated that propofol significantly reduced the viability of OS cells and promoted autophagy in a dose-dependent manner. Moreover, cell treatment with propofol significantly enhanced the protein expression levels of phosphorylated (p)-AMPK and FOXO1, while decreasing the protein levels of p-FOXO1. Furthermore, treatment with propofol significantly suppressed cell viability, migration and invasion abilities and the EMT of OS cells, and potentially promoted cell apoptosis via inducing autophagy via the AMPK/FOXO1 signaling pathway. In summary, the present study indicated that propofol potentially had an inhibitory effect on the development of OS cells via AMPK/FOXO1-mediated autophagy. These results have therefore provided an experimental basis for further studies into the therapeutic effect of propofol on OS.
Keywords: adenosine monophosphate-activated protein kinase/FOXO1 signaling pathway; autophagy; metastasis; osteosarcoma; propofol.
Copyright: © Dai et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
