Role of phloretin as a sensitizer to TRAIL-induced apoptosis in colon cancer
- PMID: 35949608
- PMCID: PMC9353883
- DOI: 10.3892/ol.2022.13441
Role of phloretin as a sensitizer to TRAIL-induced apoptosis in colon cancer
Abstract
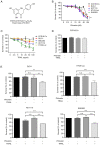
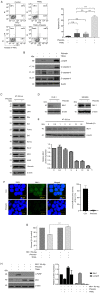
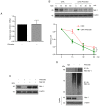
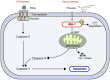
Phloretin is one of the apple polyphenols with anticancer activities. Since tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) serves important roles in inducing apoptosis, the present study examined the effect of phloretin on TRAIL-induced apoptosis in colon cancer cells. Treatment with both phloretin and TRAIL markedly suppressed the survival of cancer cells from several colon cancer cell lines compared with that of cells treated with either TRAIL or phloretin. Additionally, decreased numbers of colonies were observed following addition of phloretin and TRAIL. Furthermore, TRAIL- and phloretin-treated HT-29-Luc cells exhibited decreased luciferase activity. Increased apoptosis was observed in phloretin- and TRAIL-treated HT-29-Luc colon cancer cells, accompanying elevated levels of cleaved poly(ADP-ribose) polymerase, and caspase-3, -8 and -9. The expression levels of MCL1 apoptosis regulator BCL2 family member (Mcl-1) were decreased following addition of phloretin in colon cancer cells. In addition, overexpression of Mcl-1 in phloretin- and TRAIL-treated HT-29-Luc cells resulted in increased cell survival. Treatment of HT-29-Luc cells with a combination of cycloheximide (CHX) and phloretin led to a more prominent decrease in Mcl-1 expression compared with that in cells treated with CHX alone, while Mcl-1 expression was recovered by treatment with MG132. Binding of ubiquitin with Mcl-1 was verified using immunoprecipitation. Intraperitoneal injection of both TRAIL and phloretin into tumor xenografts was associated with a decreased tumor volume compared with that following injection with either TRAIL or phloretin. Overall, the present results suggest a synergistic effect of phloretin on TRAIL-induced apoptosis in colon cancer cells.
Keywords: BCL2 family member; MCL1 apoptosis regulator; apoptosis; colon cancer; phloretin; tumor necrosis factor-related apoptosis-inducing ligand.
Copyright: © Kim et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Induction of apoptosis in HT-29 colon cancer cells by phloretin.J Med Food. 2007 Dec;10(4):581-6. doi: 10.1089/jmf.2007.116. J Med Food. 2007. PMID: 18158826
-
Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells.J Biol Chem. 2005 Mar 18;280(11):10491-500. doi: 10.1074/jbc.M412819200. Epub 2005 Jan 6. J Biol Chem. 2005. PMID: 15637055
-
The regulatory protein GADD34 inhibits TRAIL-induced apoptosis via TRAF6/ERK-dependent stabilization of myeloid cell leukemia 1 in liver cancer cells.J Biol Chem. 2019 Apr 12;294(15):5945-5955. doi: 10.1074/jbc.RA118.006029. Epub 2019 Feb 19. J Biol Chem. 2019. PMID: 30782845 Free PMC article.
-
Regulation of tumor necrosis factor-related apoptosis-inducing ligand sensitivity in primary and transformed human keratinocytes.Cancer Res. 2000 Feb 1;60(3):553-9. Cancer Res. 2000. PMID: 10676636
-
Clitocine potentiates TRAIL-mediated apoptosis in human colon cancer cells by promoting Mcl-1 degradation.Apoptosis. 2016 Oct;21(10):1144-57. doi: 10.1007/s10495-016-1273-y. Apoptosis. 2016. PMID: 27421828
Cited by
-
Phloretin, as a Potent Anticancer Compound: From Chemistry to Cellular Interactions.Molecules. 2022 Dec 12;27(24):8819. doi: 10.3390/molecules27248819. Molecules. 2022. PMID: 36557950 Free PMC article. Review.
-
Phloridzin as a Nutraceutical for Cancer Prevention and Therapy: A Comprehensive Review of Its Mechanisms, Bioavailability Challenges and Future Applications.Food Sci Nutr. 2025 Aug 4;13(8):e70744. doi: 10.1002/fsn3.70744. eCollection 2025 Aug. Food Sci Nutr. 2025. PMID: 40761486 Free PMC article. Review.
-
Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin.Nutrients. 2022 Sep 2;14(17):3638. doi: 10.3390/nu14173638. Nutrients. 2022. PMID: 36079895 Free PMC article. Review.
-
Development and evaluation of polyacrylamide microspheres loaded with phloretin and tantalum for transcatheter arterial embolization.RSC Adv. 2023 Dec 5;13(50):35429-35434. doi: 10.1039/d3ra05841g. eCollection 2023 Nov 30. RSC Adv. 2023. PMID: 38058558 Free PMC article.
-
Combination treatment with Phloretin enhances the anti-tumor efficacy of radiotherapy in lung cancer models.Discov Oncol. 2025 May 7;16(1):685. doi: 10.1007/s12672-025-02516-0. Discov Oncol. 2025. PMID: 40335757 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
