Thromboprophylaxis for children hospitalized with COVID-19 and MIS-C
- PMID: 35949885
- PMCID: PMC9357887
- DOI: 10.1002/rth2.12780
Thromboprophylaxis for children hospitalized with COVID-19 and MIS-C
Abstract
Background: Limited data exist about effective regimens for pharmacological thromboprophylaxis in children with acute coronavirus disease 2019 (COVID-19) and multisystem inflammatory syndrome in children (MIS-C).
Objectives: Study the outcomes of institutional thromboprophylaxis protocol for primary venous thromboembolism (VTE) prevention in children hospitalized with acute COVID-19/MIS-C.
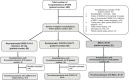
Methods: This single-center retrospective cohort study included consecutive children (aged less than 21 years) with COVID-19/MIS-C who received tailored intensity thromboprophylaxis, primarily with low-molecular-weight heparin, from April 2020 through October 2021. Thromboprophylaxis was given to those with moderate to severe disease based on the World Health Organization scale and exposure to two or more VTE risk factors. Therapeutic intensity was considered for severe illness. Clinical recovery along with D-dimer improvement determined thromboprophylaxis duration. Outcomes were incident VTEs, bleeding, and mortality.
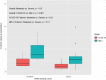
Results: Among 211 hospitalizations, 45 (21.3%) received thromboprophylaxis (COVID-19, 16; MIS-C, 29). Median age was 14.8 years (interquartile range [IQR], 8.9-16.1). Among 35 (77.8%) with severe illness, 27 (60.0%) required respiratory support, and 19 (42.2%) required an intensive care unit stay. Median hospitalization was 6 days (IQR, 5.0-10.5). Median thromboprophylaxis duration was 19 days (IQR, 6.0-31.0) with therapeutic intensity in 24 (53.3%) and prophylactic in 21 (46.7%). Outcomes were as follows: VTE, 1 (2.2%); death, 1 (2.2%, unrelated to bleeding/thrombosis); major/clinically relevant nonmajor bleeding, 0; and minor bleeding, 7 (15.5%). D-dimer was elevated in a majority at diagnosis (median, 2.3; IQR, 1.2-3.3 mg/ml fibrinogen-equivalent units) and was noninformative in assessing disease severity. D-dimer normalized at thromboprophylaxis discontinuation.
Conclusions: Our experience of using clinically directed thromboprophylaxis with tailored intensity approach for children hospitalized with COVID-19 and MIS-C favors its inclusion in current standard of care. The role of D-dimer in directing thromboprophylaxis management deserves further evaluation.
Keywords: COVID‐19; MIS‐C; anticoagulants; child; heparin; low molecular weight; thrombosis; venous thromboembolism.
© 2022 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures


References
-
- WHO COVID‐19 dashboard . https://covid19.who.int/?gclid=Cj0KCQiAj9iBBhCJARIsAE9qRtCRe5ZTOFO498tpc... (accessed April 1, 2022).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

