Retrospective Multicenter Real-Life Study on the First-Line Treatment of Classical Hodgkin Lymphoma in Argentina
- PMID: 35950206
- PMCID: PMC9358792
- DOI: 10.1007/s44228-022-00008-4
Retrospective Multicenter Real-Life Study on the First-Line Treatment of Classical Hodgkin Lymphoma in Argentina
Abstract
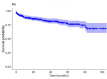
There are no data in Argentina on the response rates to first-line treatment of classical Hodgkin Lymphoma (cHL) outside clinical trials. A total of 498 patients from 7 public and private hospitals in Argentina were retrospectively examined. The median follow-up was 37.4 months (CI 95% 17.7-63.5). The median time from diagnosis to treatment was 22 days (IQR 14-42), which was significantly longer in public hospitals (49.3 (IC 95% 38.5-60.2) versus 32.5 (IC 95% 27-38); p = 0.0027). A total of 96.8% of patients were treated with ABVD.:84.3% achieved complete remission (CR) and 6.02% partial remission (PR), being the CR rate higher in private hospitals. End-of-treatment metabolic CR was achieved in 85.4% (n = 373). The interim PET scan was widely used in our cohort (70.5%; n = 351), but in only 23.3% (n = 116) was the treatment strategy response-adapted. The 5-year progression-free survival (PFS) was 76% (CI 95% 70-81). The 2 and 5-years-OS rates were 91% (CI 95% 88-94%) and 85% (CI 95% 80-89%), respectively. No differences in OS were found between public and private institutions (p = 0.27). This is one of the largest retrospective cHL cohorts reported. In Argentina ABVD is the chemotherapy regimen of choice and, although it is well tolerated, it is not exempt from toxicity. We showed that early initiation of treatment impacts the induction results. Although the use of PET scan is widespread, only a minority of patients was treated with respons- adapted strategies. The use of PET-guided treatment is strongly encouraged.
Keywords: Hodgkin disease; Pet-adapted; Real world evidence; Survival; Toxicity.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestNone of the authors have anything to disclose with respect to this article.
Figures
References
-
- Muruthy G, Szabo A, Hamadani M, Fenske T, Shah N. Contemporary Outcomes for Advanced-Stage Classical Hodgkin Lymphoma in the U.S.: Analysis of Surveillance, Epidemiology, and End Results Database Contemporary Outcomes for Advanced-Stage Classical Hodgkin Lymphoma in the U.S.: Analysis of Surveillance, Epidemiology, and End Results Database. Oncologist. 2019;24:1488–1494. doi: 10.1634/theoncologist.2019-0172. - DOI - PMC - PubMed
-
- Globocan . International Agency for research on cancer. WHO; 2018.
LinkOut - more resources
Full Text Sources
Research Materials