Prevalence and prognostic implications of reduced left ventricular ejection fraction among patients with STEMI in India
- PMID: 35950269
- PMCID: PMC9773730
- DOI: 10.1002/ehf2.14055
Prevalence and prognostic implications of reduced left ventricular ejection fraction among patients with STEMI in India
Abstract
Aims: To describe clinical characteristics and outcomes for those with STEMI and reduced left ventricular ejection fraction (LVEF) in low-income and middle-income countries (LMICs).
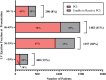
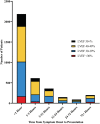
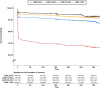
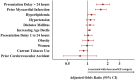
Methods and results: Adults presenting with STEMI to two government-owned tertiary care centres in Delhi, India were prospectively enrolled in the North India ST-elevation myocardial infarction (NORIN-STEMI) registry. LVEF was evaluated at presentation and clinical characteristics were compared across LVEF categories. Overall, 3597 patients were included, of whom 468 (13%) had LVEF >50%, 1482 (41%) had mildly reduced LVEF (40-49%), 1357 (38%) had moderately reduced LVEF (30-39%), and 290 (8%) had severely reduced LVEF (<30%). Presentation delay >24 h, prior MI, and hyperlipidaemia were associated with decreasing LVEF category. Although most patients with reduced LVEF were discharged on appropriate guideline-directed therapies, adherence at 1 year was low (ACE inhibitor/ARB 91% to 41%, beta blocker 98% to 78%, aldosterone receptor antagonist 69% to 6%). After multivariable adjustment, a Cox regression model showed moderately reduced LVEF (HR 1.77, 95% CI 1.20, 2.60) and severely reduced LVEF (HR 3.63, 95% CI 2.41, 5.48) were associated with increased risk of all-cause mortality compared with LVEF ≥50%.
Conclusions: On presentation for STEMI, almost 90% of NORIN-STEMI participants had at least mildly reduced LVEF and almost half had LVEF <40%. Patients with LVEF <40% had significantly higher risk of mortality at 1 year, and adherence to guideline-directed therapies at 1 year was poor. Systematic initiatives to improve access to timely revascularization and guideline-directed therapies are essential in advancing STEMI care in LMICs.
Keywords: NORIN-STEMI; ST-elevation myocardial infarction; atherosclerotic cardiovascular disease; heart failure; left ventricular dysfunction.
© 2022 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Dr. Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, speaker engagements with Novartis, AstraZeneca, and Roche Diagnostics, and participates on clinical trial committees for studies sponsored by Galmed, Impulse Dynamics, Bayer AG, Novartis, and Occlutech. Dr. Bhatt discloses the following relationships ‐ Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News,
Figures

Similar articles
-
Impact of Adherence to Beta-Blockers in Patients With All-Comers ST-Segment Elevation Myocardial Infarction and According to Left Ventricular Ejection Fraction at Discharge: Results From the Real-World Registry FAST-STEMI.J Cardiovasc Pharmacol. 2024 Dec 1;84(6):581-589. doi: 10.1097/FJC.0000000000001627. J Cardiovasc Pharmacol. 2024. PMID: 39259210
-
Short-term efficacy of angiotensin receptor-neprilysin inhibitor treatment in patients with ST-segment elevation myocardial infarction with reduced ejection fraction after primary percutaneous coronary intervention: a propensity score matching study.BMC Cardiovasc Disord. 2022 Nov 4;22(1):463. doi: 10.1186/s12872-022-02906-0. BMC Cardiovasc Disord. 2022. PMID: 36333668 Free PMC article.
-
Impact of renin-angiotensin system blockade on the prognosis of acute coronary syndrome based on left ventricular ejection fraction.Rev Esp Cardiol (Engl Ed). 2020 Feb;73(2):114-122. doi: 10.1016/j.rec.2019.02.012. Epub 2019 May 17. Rev Esp Cardiol (Engl Ed). 2020. PMID: 31105064 English, Spanish.
-
Efficacy of Long-Term Oral Beta-Blocker Therapy in Patients Who Underwent Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction With Preserved Left Ventricular Ejection Fraction: A Systematic Review and Meta-analysis.J Cardiovasc Pharmacol. 2021 Jan 1;77(1):87-93. doi: 10.1097/FJC.0000000000000922. J Cardiovasc Pharmacol. 2021. PMID: 33136764
-
Does Oral Beta-Blocker Therapy Improve Long-Term Survival in ST-Segment Elevation Myocardial Infarction With Preserved Systolic Function? A Meta-Analysis.J Cardiovasc Pharmacol Ther. 2016 May;21(3):280-5. doi: 10.1177/1074248415608011. Epub 2015 Sep 29. J Cardiovasc Pharmacol Ther. 2016. PMID: 26424094 Review.
Cited by
-
Comparative evaluation of machine learning models versus TIMI score in ST-segment-elevation myocardial infarction patients.Indian Heart J. 2025 May-Jun;77(3):133-141. doi: 10.1016/j.ihj.2025.03.010. Epub 2025 Mar 27. Indian Heart J. 2025. PMID: 40157569 Free PMC article.
-
Shorter Door-to-ECG Time Is Associated with Improved Mortality in STEMI Patients.J Clin Med. 2024 Apr 30;13(9):2650. doi: 10.3390/jcm13092650. J Clin Med. 2024. PMID: 38731180 Free PMC article.
-
Influencing factors and prognostic value of left ventricular systolic dysfunction in patients with complete occlusion of the left anterior descending artery reperfused by primary percutaneous coronary intervention.BMC Cardiovasc Disord. 2023 Jul 10;23(1):344. doi: 10.1186/s12872-023-03341-5. BMC Cardiovasc Disord. 2023. PMID: 37430213 Free PMC article.
References
-
- Gheorghiade M, Bonow RO. Chronic heart failure in the United States: A manifestation of coronary artery disease. Circulation. 1998; 97: 282–289. - PubMed
-
- Lund LH, Mancini D. Heart failure in women. Med Clin North Am. 2004; 88: 1321–1345. - PubMed
-
- White HD, Cross DB, Elliott JM, Norris RM, Yee TW. Long‐term prognostic importance of patency of the infarct‐related coronary artery after thrombolytic therapy for acute myocardial infarction. Circulation. 1994; 89: 61–67. - PubMed
-
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur Heart J. 2016; 37: 3232–3245. - PubMed
-
- Jernberg T, Omerovic E, Hamilton E, Lindmark K, Desta L, Alfredsson J, Lundberg A, Kellerth T, Erlinge D. Prevalence and prognostic impact of left ventricular systolic dysfunction after acute myocardial infarction. Eur Heart J [Internet]. 2020. [cited 2021 Nov 4];41. Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/ehjci/ehaa946.179... (Accessed 11/22/21). - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

