A phase II trial of regorafenib in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC024 trial results
- PMID: 35950293
- PMCID: PMC9883574
- DOI: 10.1002/cam4.5044
A phase II trial of regorafenib in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC024 trial results
Abstract
Background: Regorafenib is one of several FDA-approved cancer therapies targeting multiple tyrosine kinases. However, there are few subtype-specific data regarding kinase inhibitor activity in sarcomas. We report results of a single arm, phase II trial of regorafenib in advanced Ewing family sarcomas.
Methods: Patients with metastatic Ewing family sarcomas (age ≥ 18, ECOG 0-2, good organ function) who had received at least one line of therapy and experienced progression within 6 months of registration were eligible. Prior kinase inhibitors were not allowed. The initial dose of regorafenib was 160 mg oral days 1-21 of a 28-day cycle. The primary endpoint was estimating progression-free rate (PFR) at 8 weeks employing RECIST 1.1.
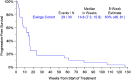
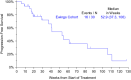
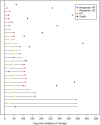
Results: Thirty patients (median age, 32 years; 33% women [10 patients]; bone primary, 40%; extraskeletal primary, 60%) enrolled at 14 sites. The most common grade 3 or higher toxicities were hypophosphatemia (5 grade 3, 1 grade 4), hypertension (2 grade 3), elevated ALT (2 grade 3). Sixteen patients required dose reductions, most often for hypophosphatemia (n = 7 reductions in 6 patients); two stopped regorafenib for toxicity. There was one death unrelated to treatment in the 30-day post-study period. Median progression-free survival (PFS) was 14.8 weeks (95% CI 7.3-15.9); PFR at 8 weeks by Kaplan-Meier analysis was 63% (95% CI 46-81%). The RECIST 1.1 response rate was 10%. Median OS was 53 weeks (95% CI 37-106 weeks).
Conclusions: Regorafenib has modest activity in the Ewing family sarcomas. Toxicity was similar to that seen in approval studies.
Keywords: (5): CIC-DUX4; Ewing sarcoma; clinical trial; regorafenib.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The following conflicts of interest are reported.
Figures
References
-
- Brennan MF, Antonescu C, Maki RG. Sarcomas more common in children. Management of Soft Tissue Sarcoma. Springer; 2013. ISBN 978‐1‐4614‐5003‐0: 2210‐250.
-
- Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: the memorial Sloan‐Kettering experience. Pediatr Blood Cancer. 2009;53(6):1029‐1034. - PubMed
-
- Wagner LM, McAllister N, Goldsby RE, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48(2):132‐139. - PubMed
-
- Farhat R, Raad R, Khoury NJ, et al. Cyclophosphamide and topotecan as first‐line salvage therapy in patients with relapsed Ewing sarcoma at a single institution. J Pediatr Hematol Oncol. 2013;35(5):356‐360. - PubMed
-
- Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jürgens H. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer. 2006;47(6):795‐800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous