An accurate fully automated panel of plasma biomarkers for Alzheimer's disease
- PMID: 35950735
- PMCID: PMC9918613
- DOI: 10.1002/alz.12751
An accurate fully automated panel of plasma biomarkers for Alzheimer's disease
Abstract
Introduction: There is a great need for fully automated plasma assays that can measure amyloid beta (Aβ) pathology and predict future Alzheimer's disease (AD) dementia.
Methods: Two cohorts (n = 920) were examined: Panel A+ (n = 32 cognitively unimpaired [CU], n = 106 mild cognitive impairment [MCI], and n = 89 AD) and BioFINDER-1 (n = 461 CU, n = 232 MCI). Plasma Aβ42/Aβ40, phosphorylated tau (p-tau)181, two p-tau217 variants, ApoE4 protein, neurofilament light, and GFAP were measured using Elecsys prototype immunoassays.
Results: The best biomarker for discriminating Aβ-positive versus Aβ-negative participants was Aβ42/Aβ40 (are under the curve [AUC] 0.83-0.87). Combining Aβ42/Aβ40, p-tau181, and ApoE4 improved the AUCs significantly (0.90 to 0.93; P< 0.01). Adding additional biomarkers had marginal effects (ΔAUC ≤0.01). In BioFINDER, p-tau181, p-tau217, and ApoE4 predicted AD dementia within 6 years in CU (AUC 0.88) and p-tau181, p-tau217, and Aβ42/Aβ40 in MCI (AUC 0.87).
Discussion: The high accuracies for Aβ pathology and future AD dementia using fully automated instruments are promising for implementing plasma biomarkers in clinical trials and clinical routine.
Keywords: Alzheimer's disease; Elecsys; amyloid beta; apolipoprotein E; area under the curve; blood; cerebrospinal fluid; clinical practice; cognitively unimpaired; diagnostics; fully automated instruments; glial fibrillary acidic protein; immunoassays; implementation; mild cognitive impairment; neurofilament light; phosphorylated tau; plasma; prediction; prognostics.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Figures


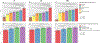


References
-
- Hansson O, Biomarkers for neurodegenerative diseases. Nat Med, 2021. 27(6): p. 954–963. - PubMed
-
- Palmqvist S, et al. , Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat Med, 2021. 27(6): p. 1034–1042. - PubMed
-
- Karikari TK, et al. , Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol, 2020. 19(5): p. 422–433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

