Mumps serological surveillance following 10 years of a one-dose mumps-containing-vaccine policy in Fujian Province, China
- PMID: 35950847
- PMCID: PMC9746472
- DOI: 10.1080/21645515.2022.2096375
Mumps serological surveillance following 10 years of a one-dose mumps-containing-vaccine policy in Fujian Province, China
Abstract
Background: Since 2008, Fujian province provided measles-rubella (MR) vaccine at 8 months followed by measles-mumps-rubella (MMR) vaccine at 18 months a one-dose mumps-containing-vaccine (MuCV) schedule. Several mumps outbreaks have occurred recently in Fujian. Serological surveillance can assess population immunity to mumps and identify risk factors for mumps.
Methods: We conducted a cross-sectional serosurvey of mumps IgG antibodies in the general population of Fujian Province in 2018 and compare results with a similar study conducted in 2009, when the routine schedule had no MuCV. We analyzed changes in mumps epidemiology after implementation of a one-dose MuCV vaccination strategy.
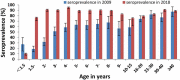
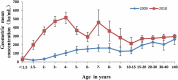
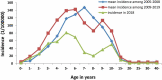
Results: Mumps seroprevalence was 78.6% (95% CI: 77.4-79.8), and the geometric mean concentration (GMC) of mumps antibodies was 245.8 IU/ml (95% CI:237.3-255.1). MuCV vaccination at 18 months resulted in increased seroprevalence and GMCs. Seroprevalence and GMCs varied by age, gender, and number of doses received. Except for children under 18 months, seroprevalence and GMCs were lowest among 10-15-year-olds. Each year after introduction of the one-dose MuCV vaccination policy, the highest incidence of mumps was among 4-6-year-olds and 9-15-year-olds, gradually shifting to older age groups.
Conclusion: A one-dose mumps-containing vaccine schedule does not provide sustained and stable mumps immunity in Fujian. To reduce the risk of mumps, we recommend supplementary vaccination of children without a history of receiving at least one MuCV dose or who are seronegative at 10-15 years of age.
Keywords: Geometric Mean Concentration (GMC); Mumps; measles-mumps-rubella vaccine (MMR); seroprevalence; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
