Amyloid Aggregates Are Localized to the Nonadherent Detached Fraction of Aging Streptococcus mutans Biofilms
- PMID: 35950854
- PMCID: PMC9431626
- DOI: 10.1128/spectrum.01661-22
Amyloid Aggregates Are Localized to the Nonadherent Detached Fraction of Aging Streptococcus mutans Biofilms
Abstract
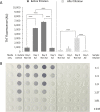
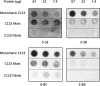
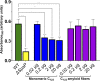
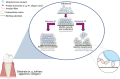
The number of bacterial species recognized to utilize purposeful amyloid aggregation within biofilms continues to grow. The oral pathogen Streptococcus mutans produces several amyloidogenic proteins, including adhesins P1 (also known as AgI/II, PAc) and WapA, whose truncation products, namely, AgII and AgA, respectively, represent the amyloidogenic moieties. Amyloids demonstrate common biophysical properties, including recognition by Thioflavin T (ThT) and Congo red (CR) dyes that bind to the cross β-sheet quaternary structure of amyloid aggregates. Previously, we observed amyloid formation to occur only after 60 h or more of S. mutans biofilm growth. Here, we extend those findings to investigate where amyloid is detected within 1- and 5-day-old biofilms, including within tightly adherent compared with those in nonadherent fractions. CR birefringence and ThT uptake demonstrated amyloid within nonadherent material removed from 5-day-old cultures but not within 1-day-old or adherent samples. These experiments were done in conjunction with confocal microscopy and immunofluorescence staining with AgII- and AgA-reactive antibodies, including monoclonal reagents shown to discriminate between monomeric protein and amyloid aggregates. These results also localized amyloid primarily to the nonadherent fraction of biofilms. Lastly, we show that the C-terminal region of P1 loses adhesive function following amyloidogenesis and is no longer able to competitively inhibit binding of S. mutans to its physiologic substrate, salivary agglutinin. Taken together, our results provide new evidence that amyloid aggregation negatively impacts the functional activity of a widely studied S. mutans adhesin and are consistent with a model in which amyloidogenesis of adhesive proteins facilitates the detachment of aging biofilms. IMPORTANCE Streptococcus mutans is a keystone pathogen and causative agent of human dental caries, commonly known as tooth decay, the most prevalent infectious disease in the world. Like many pathogens, S. mutans causes disease in biofilms, which for dental decay begins with bacterial attachment to the salivary pellicle coating the tooth surface. Some strains of S. mutans are also associated with bacterial endocarditis. Amyloid aggregation was initially thought to represent only a consequence of protein mal-folding, but now, many microorganisms are known to produce functional amyloids with biofilm environments. In this study, we learned that amyloid formation diminishes the activity of a known S. mutans adhesin and that amyloid is found within the nonadherent fraction of older biofilms. This finding suggests that the transition from adhesin monomer to amyloid facilitates biofilm detachment. Knowing where and when S. mutans produces amyloid will help in developing therapeutic strategies to control tooth decay and other biofilm-related diseases.
Keywords: Streptococcus mutans; adhesins; amyloid; biofilms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

