Clot-targeted magnetic hyperthermia permeabilizes blood clots to make them more susceptible to thrombolysis
- PMID: 35950914
- PMCID: PMC9826519
- DOI: 10.1111/jth.15846
Clot-targeted magnetic hyperthermia permeabilizes blood clots to make them more susceptible to thrombolysis
Abstract
Background: Thrombolysis is a frontline treatment for stroke, which involves the application of tissue plasminogen activator (tPA) to trigger endogenous clot-degradation pathways. However, it is only effective within 4.5 h of symptom onset because of clot contraction preventing tPA permeation into the clot. Magnetic hyperthermia (MH) mediated by tumor-targeted magnetic nanoparticles is used to treat cancer by using local heat generation to trigger apoptosis of cancer cells.
Objectives: To develop clot-targeting magnetic nanoparticles to deliver MH to the surface of human blood clots, and to assess whether this can improve the efficacy of thrombolysis of contracted blood clots.
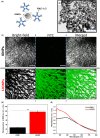
Methods: Clot-targeting magnetic nanoparticles were developed by functionalizing iron oxide nanoparticles with an antibody recognizing activated integrin αIIbβ3 (PAC-1). The magnetic properties of the PAC-1-tagged magnetic nanoparticles were characterized and optimized to deliver clot-targeted MH.
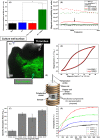
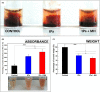
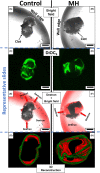
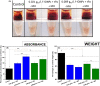
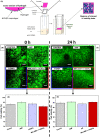
Results: Clot-targeted MH increases the efficacy of tPA-mediated thrombolysis in contracted human blood clots, leading to a reduction in clot weight. MH increases the permeability of the clots to tPA, facilitating their breakdown. Scanning electron microscopy reveals that this effect is elicited through enhanced fibrin breakdown and triggering the disruption of red blood cells on the surface of the clot. Importantly, endothelial cells viability in a three-dimensional blood vessel model is unaffected by exposure to MH.
Conclusions: This study demonstrates that clot-targeted MH can enhance the thrombolysis of contracted human blood clots and can be safely applied to enhance the timeframe in which thrombolysis is effective.
Keywords: blood clot permeation; clot-targeted magnetic hyperthermia; functionalized iron oxide nanoparticles; stroke; thrombolysis.
© 2022 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures

Similar articles
-
Fibrin-targeted perfluorocarbon nanoparticles for targeted thrombolysis.Nanomedicine (Lond). 2007 Aug;2(4):533-43. doi: 10.2217/17435889.2.4.533. Nanomedicine (Lond). 2007. PMID: 17716136
-
Differences in clot preparation determine outcome of recombinant tissue plasminogen activator treatment in experimental thromboembolic stroke.Stroke. 2003 Aug;34(8):2019-24. doi: 10.1161/01.STR.0000080941.73934.30. Epub 2003 Jul 3. Stroke. 2003. PMID: 12843350
-
Effect of low frequency ultrasound on combined rt-PA and eptifibatide thrombolysis in human clots.Thromb Res. 2009;123(3):528-36. doi: 10.1016/j.thromres.2008.05.011. Epub 2008 Jul 10. Thromb Res. 2009. PMID: 18619651 Free PMC article.
-
Novel and emerging therapies: thrombus-targeted fibrinolysis.Semin Thromb Hemost. 2013 Feb;39(1):48-58. doi: 10.1055/s-0032-1328935. Epub 2012 Oct 3. Semin Thromb Hemost. 2013. PMID: 23034825 Review.
-
Recent advances in targeted delivery of tissue plasminogen activator for enhanced thrombolysis in ischaemic stroke.J Drug Target. 2018 Feb;26(2):95-109. doi: 10.1080/1061186X.2017.1365874. Epub 2017 Aug 25. J Drug Target. 2018. PMID: 28796540 Review.
Cited by
-
Spermatozoon-propelled microcellular submarines combining innate magnetic hyperthermia with derived nanotherapies for thrombolysis and ischemia mitigation.J Nanobiotechnology. 2024 Aug 8;22(1):470. doi: 10.1186/s12951-024-02716-w. J Nanobiotechnology. 2024. PMID: 39118029 Free PMC article.
-
Near-infrared-II photoacoustic imaging and photo-triggered synergistic treatment of thrombosis via fibrin-specific homopolymer nanoparticles.Nat Commun. 2023 Oct 28;14(1):6881. doi: 10.1038/s41467-023-42691-8. Nat Commun. 2023. PMID: 37898604 Free PMC article.
-
Progress of nanomaterials in the treatment of thrombus.Drug Deliv Transl Res. 2024 May;14(5):1154-1172. doi: 10.1007/s13346-023-01478-6. Epub 2023 Nov 25. Drug Deliv Transl Res. 2024. PMID: 38006448 Review.
-
Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery.Bioact Mater. 2025 Jul 26;53:591-629. doi: 10.1016/j.bioactmat.2025.07.033. eCollection 2025 Nov. Bioact Mater. 2025. PMID: 40761549 Free PMC article. Review.
-
Magnetic coagulometry: towards a new nanotechnological tool for ex vivo monitoring coagulation in human whole blood.Nanoscale. 2024 Feb 15;16(7):3534-3548. doi: 10.1039/d3nr02593d. Nanoscale. 2024. PMID: 38285061 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 207617/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- NC/R001642/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- NC/R001642/1/BHF_/British Heart Foundation/United Kingdom
- 218671/Z/19/Z/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

