Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases
- PMID: 35951483
- PMCID: PMC9576172
- DOI: 10.1152/physrev.00062.2021
Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases
Abstract
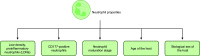
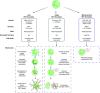
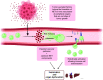
Neutrophil extracellular trap (NET) formation, first described in 2004 as a previously unknown strategy of neutrophils to fight microbes, has attracted an increasing interest in the research community. NETs are formed when neutrophils externalize their decondensed chromatin together with content from their azurophilic granules. In addition to their role in defense against microbes, NETs have been implicated as mediators of pathology in sterile inflammation, such as cancer and autoimmunity, and their potential as therapeutic targets is actively explored. However, targeting of NETs is challenging since the beneficial effects of their removal need to be balanced against the potential harmful loss of their function in microbial defense. Moreover, depending on the stimuli or species, NETs can be formed via distinct mechanisms and are not always made up of the same components, making direct comparisons between various studies challenging. This review focuses on the role of NETs in cancer-associated pathology, such as thrombosis, organ dysfunction, and metastasis. Different strategies to target NETs, by either preventing their formation or degrading existing ones, are also discussed.
Keywords: cancer-associated pathology; inflammation; neutrophil extracellular traps; thrombosis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

