Preoperative BRAFV600E mutation detection in thyroid carcinoma by immunocytochemistry
- PMID: 35951496
- PMCID: PMC9804421
- DOI: 10.1111/apm.13267
Preoperative BRAFV600E mutation detection in thyroid carcinoma by immunocytochemistry
Abstract
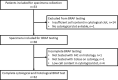
The BRAFV600E (BRAF) mutation is present in 40-50% of papillary thyroid carcinomas (PTC) and has been associated with more aggressive clinicopathological characteristics of PTC. The aim of this study was to evaluate different methods for preoperative identification of the BRAF mutation in PTC using cytological and histological specimens. Prospectively collected preoperative cytological clots from patients with suspected PTC were tested with BRAF immunocytochemistry (ICC) and the Cobas Test (PCR). In addition, histological specimens were tested with BRAF immunohistochemistry (IHC) and the Cobas Test. All nodules were histologically examined. Fifty-three patients were included in the study. Complete mutation testing was available in 32 patients. The main reason for exclusion was insufficient cell content in the cytological specimen. Twenty-seven nodules were histologically diagnosed as PTC, and 41% (n = 11) of PTCs were BRAF ICC positive. All non-PTC nodules were negative by BRAF ICC. In 26 nodules, all four BRAF tests were concordant, while discordant test results were found in six nodules. ICC was in accordance with the consensus BRAF status in five of these nodules, while BRAF status was undetermined in one nodule. BRAF ICC showed high concordance with the Cobas Test and a low rate of false negative stain. These results indicate that BRAF ICC may be a feasible method for preoperative detection of the BRAFV600E mutation in patients with PTC.
Keywords: BRAF; Thyroid; diagnosis; immunocytochemistry; thyroid carcinoma.
© 2022 The Authors. APMIS published by John Wiley & Sons Ltd on behalf of Scandinavian Societies for Pathology, Medical Microbiology and Immunology.
Conflict of interest statement
The authors have no conflict of interests to declare that are relevant to the content of this article.
Figures


References
-
- Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367(8):705–15. - PubMed
-
- Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: review and current state. Cancer. 2018;124(5):888–98. - PubMed
-
- Boufraqech M, Patel D, Xiong Y, Kebebew E. Diagnosis of thyroid cancer: state of art. Expert Opin Med Diagn. 2013;7(4):331–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

