Host genotype controls ecological change in the leaf fungal microbiome
- PMID: 35951523
- PMCID: PMC9371330
- DOI: 10.1371/journal.pbio.3001681
Host genotype controls ecological change in the leaf fungal microbiome
Abstract
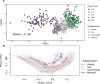
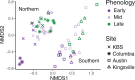
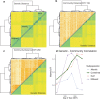
Leaf fungal microbiomes can be fundamental drivers of host plant success, as they contain pathogens that devastate crop plants and taxa that enhance nutrient uptake, discourage herbivory, and antagonize pathogens. We measured leaf fungal diversity with amplicon sequencing across an entire growing season in a diversity panel of switchgrass (Panicum virgatum). We also sampled a replicated subset of genotypes across 3 additional sites to compare the importance of time, space, ecology, and genetics. We found a strong successional pattern in the microbiome shaped both by host genetics and environmental factors. Further, we used genome-wide association (GWA) mapping and RNA sequencing to show that 3 cysteine-rich receptor-like kinases (crRLKs) were linked to a genetic locus associated with microbiome structure. We confirmed GWAS results in an independent set of genotypes for both the internal transcribed spacer (ITS) and large subunit (LSU) ribosomal DNA markers. Fungal pathogens were central to microbial covariance networks, and genotypes susceptible to pathogens differed in their expression of the 3 crRLKs, suggesting that host immune genes are a principal means of controlling the entire leaf microbiome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
The changing influence of host genetics on the leaf fungal microbiome throughout plant development.PLoS Biol. 2022 Aug 12;20(8):e3001748. doi: 10.1371/journal.pbio.3001748. eCollection 2022 Aug. PLoS Biol. 2022. PMID: 35960709 Free PMC article.
References
-
- Toju H, Vannette RL, Gauthier M-PL, Dhami MK, Fukami T. Priority effects can persist across floral generations in nectar microbial metacommunities. Oikos. 2018;127:345–52.
-
- Barton LL, McLean RJC. Environmental Microbiology and Microbial Ecology. John Wiley & Sons; 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

