Glial fibrillary acidic protein in cerebrospinal fluid of patients with spinal muscular atrophy
- PMID: 35951535
- PMCID: PMC9463944
- DOI: 10.1002/acn3.51645
Glial fibrillary acidic protein in cerebrospinal fluid of patients with spinal muscular atrophy
Abstract
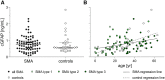
Objective: Activated astroglia is involved in the pathophysiology of neurodegenerative diseases and has also been described in animal models of spinal muscular atrophy (SMA). Given the urgent need of biomarkers for treatment monitoring of new RNA-modifying and gene replacement therapies in SMA, we examined glial fibrillary acidic protein concentrations in cerebrospinal fluid (cGFAP) as a marker of astrogliosis in SMA.
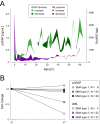
Methods: 58 adult patients and 21 children with genetically confirmed 5q-associated SMA from four German motor neuron disease specialist care centers and 30 age- and sex-matched controls were prospectively included in this study. cGFAP was measured and correlated to motor performance and disease severity. Additionally, we compared cGFAP with neurofilament light chain concentrations in cerebrospinal fluid (cNfL).
Results: cGFAP concentrations did not differ from controls but showed higher levels in more severely affected patients after adjustment for patients' age. Normalized cNfL values were associated with disease severity. Within 14 months of nusinersen treatment, cGFAP concentrations did not change, while cNfL decreased significantly.
Interpretation: cGFAP is not an outstanding biomarker in SMA, but might support the hypothesis that glial activation is involved in SMA pathology. Unlike previously suggested, cNfL may be a promising biomarker also in adult patients with SMA, which should be subject to further investigations.
© 2022 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
MF reports non‐financial support from Biogen outside the submitted work.
PS reports no disclosures. CDW has received honoraria from Biogen as an advisory board member and for lectures and as a consultant and advisory board member from Hoffmann‐La Roche. She also received travel expenses from Biogen. OSK has received honoraria as a speaker/consultant and/or funding for travel expenses from the German Neuromuscular Society “Deutsche Gesellschaft fuer Muskelkranke” (DGM e.V.), Novartis, Biogen GmbH, Biermann Verlag GmbH, MK + S ‐ Medizin, Kommunikation & Service GmbH, and the Jain Foundation, outside the submitted work; and research support from the DGM e.V., outside the submitted work. AO has received honoraria from Biogen for lectures. SP has received grants from the German Neuromuscular Society, the Federal Ministry of Education and Research, the German Israeli Foundation for Scientific Research and Development, and the EU Joint Programme for Neurodegenerative Disease Research; and other support from Cytokinetics, Desitin Pharma, Biogen, Novartis, and Teva outside of the submitted work. JCK has received payment for consultation and advisory board participation from Biogen, Hoffmann‐La Roche and AveXis. KR has no conflicts of interest to declare that are relevant to the content of this article. AHu has no conflicts of interest to declare that are relevant to the content of this article. HT funding for research projects, lectures, and travel from Alexion, Bayer, Biogen, Celgene/Bristol‐Myers‐Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Roche, Sanofi/Genzyme, Siemens and Teva, and received research support from Chemische Fabrik Karl Bucher GmbH, German Multiple Sclerosis Society (DMSG), and the German Ministry for Education and Research (BMBF). BW declares honoraria for lectures, travel support for meetings, and advisory board participation for Biogen, Novartis and Roche. BF has no conflicts of interest to declare that are relevant to the content of this article. ACL has received personal fees from AB Science, Biogen, Cytokinetics, GlaxoSmithKline, Orion Pharma, Novartis, Tau Rx Therapeutics, Teva, Mitsubishi, and Hoffmann‐La Roche outside of the submitted work. MO has no conflicts of interest to declare that are relevant to the content of this article. AH received royalties from BIOGEN and DESITIN as an advisory board member. RG has received honoraria from Biogen as an advisory board member and for lectures and as a consultant and advisory board member from Hofmann‐La Roche. He also received travel expenses and research support from Biogen.
Figures


References
-
- Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy‐determining gene. Cell. 1995;80(1):155‐165. - PubMed
-
- Park GH, Maeno‐Hikichi Y, Awano T, Landmesser LT, Monani UR. Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. J Neurosci. 2010;30(36):12005‐12019. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
