Changes in Circulating Tumor DNA Reflect Clinical Benefit Across Multiple Studies of Patients With Non-Small-Cell Lung Cancer Treated With Immune Checkpoint Inhibitors
- PMID: 35952319
- PMCID: PMC9384957
- DOI: 10.1200/PO.21.00372
Changes in Circulating Tumor DNA Reflect Clinical Benefit Across Multiple Studies of Patients With Non-Small-Cell Lung Cancer Treated With Immune Checkpoint Inhibitors
Erratum in
-
Erratum: Changes in Circulating Tumor DNA Reflect Clinical Benefit Across Multiple Studies of Patients With Non-Small-Cell Lung Cancer Treated With Immune Checkpoint Inhibitors.JCO Precis Oncol. 2023 Apr;7:e2300144. doi: 10.1200/PO.23.00144. JCO Precis Oncol. 2023. PMID: 37053538 Free PMC article. No abstract available.
Abstract
Purpose: As immune checkpoint inhibitors (ICI) become increasingly used in frontline settings, identifying early indicators of response is needed. Recent studies suggest a role for circulating tumor DNA (ctDNA) in monitoring response to ICI, but uncertainty exists in the generalizability of these studies. Here, the role of ctDNA for monitoring response to ICI is assessed through a standardized approach by assessing clinical trial data from five independent studies.
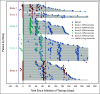
Patients and methods: Patient-level clinical and ctDNA data were pooled and harmonized from 200 patients across five independent clinical trials investigating the treatment of patients with non-small-cell lung cancer with programmed cell death-1 (PD-1)/programmed death ligand-1 (PD-L1)-directed monotherapy or in combination with chemotherapy. CtDNA levels were measured using different ctDNA assays across the studies. Maximum variant allele frequencies were calculated using all somatic tumor-derived variants in each unique patient sample to correlate ctDNA changes with overall survival (OS) and progression-free survival (PFS).
Results: We observed strong associations between reductions in ctDNA levels from on-treatment liquid biopsies with improved OS (OS; hazard ratio, 2.28; 95% CI, 1.62 to 3.20; P < .001) and PFS (PFS; hazard ratio 1.76; 95% CI, 1.31 to 2.36; P < .001). Changes in the maximum variant allele frequencies ctDNA values showed strong association across different outcomes.
Conclusion: In this pooled analysis of five independent clinical trials, consistent and robust associations between reductions in ctDNA and outcomes were found across multiple end points assessed in patients with non-small-cell lung cancer treated with an ICI. Additional tumor types, stages, and drug classes should be included in future analyses to further validate this. CtDNA may serve as an important tool in clinical development and an early indicator of treatment benefit.
Figures


References
-
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. - PubMed
-
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. - PubMed
-
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. - PubMed
-
- Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1. J Clin Oncol. 2020;38:9500–9500.
-
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

