Forgiveness Is the Attribute of the Strong: Nonadherence and Regimen Shortening in Drug-sensitive Tuberculosis
- PMID: 35952354
- PMCID: PMC9893326
- DOI: 10.1164/rccm.202201-0144OC
Forgiveness Is the Attribute of the Strong: Nonadherence and Regimen Shortening in Drug-sensitive Tuberculosis
Abstract
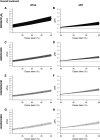
Rationale: "Forgiveness" charts the ability of a drug or regimen to withstand nonadherence without negative clinical consequences. Objectives: We aimed to determine the influence of regimen length, regimen drugs, and dosing, and when during treatment nonadherence occurs on the forgiveness of antituberculosis regimens. Methods: Using data from three randomized controlled trials comparing experimental 4-month regimens for drug-sensitive tuberculosis with the standard 6-month regimen, we used generalized linear models to examine how the risk of a negative composite outcome changed as dose-taking decreased. The percentage of doses taken and the absolute number of doses missed were calculated during the intensive and continuation phases of treatment, and overall. A mediation analysis was undertaken to determine how much the association between intensive phase dose-taking and the negative composite outcome was mediated through continuation phase dose-taking. Measurements and Main Results: Forgiveness of the 4- and 6-month regimens did not differ for any treatment period. Importantly, 4-month regimens were no less forgiving of small numbers of absolute missed doses than the 6-month regimen (e.g., for 3-7 missed doses vs. no missed doses [baseline], 6-month regimen adjusted risk ratio 1.65 [95% confidence interval, 0.80-3.41] and 4-month regimens 1.80 [1.33-2.45]). No 4-month regimen was conclusively more forgiving than another. We found evidence of mediation by continuation phase dose-taking on the intensive phase dose-taking and negative composite outcome relationship. Conclusions: With the current appetite for, and progress toward, shorter drug-sensitive tuberculosis regimens worldwide, we offer reassurance that shorter regimens are not necessarily less forgiving of nonadherence. Given the importance of continuation phase adherence, patient support during this period should not be neglected.
Keywords: adherence; forgiveness; nonadherence; treatment; tuberculosis.
Figures




Comment in
-
To Err Is Human, to Forgive Is Pharmacodynamic.Am J Respir Crit Care Med. 2023 Jan 15;207(2):127-129. doi: 10.1164/rccm.202208-1629ED. Am J Respir Crit Care Med. 2023. PMID: 36165624 Free PMC article. No abstract available.
References
-
- Iseman MD. Tuberculosis therapy: past, present and future. Eur Respir J Suppl . 2002;36:87s–94s. - PubMed
-
- Lee A, Xie YL, Barry CE, Chen RY. Current and future treatments for tuberculosis. BMJ . 2020;368:m216. - PubMed
-
- Zumla AI, Gillespie SH, Hoelscher M, Philips PP, Cole ST, Abubakar I, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis . 2014;14:327–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

