Humoral response to COVID-19 vaccination in MS patients on disease modifying therapy: Immune profiles and clinical outcomes
- PMID: 35952457
- PMCID: PMC9330583
- DOI: 10.1016/j.msard.2022.104079
Humoral response to COVID-19 vaccination in MS patients on disease modifying therapy: Immune profiles and clinical outcomes
Abstract
Background: Patients with multiple sclerosis (MS) on some disease modifying therapies (DMTs), particularly anti-CD20 and sphingosine-1-phosphate (S1P) modulators, are at increased risk of severe Coronavirus Disease 19 (COVID-19) and death. COVID-19 vaccinations are effective in preventing infection and severe disease, but humoral response to vaccination and outcomes of COVID-19 infection after vaccination in MS patients on DMTs remain less understood.
Methods: In this retrospective single-center study, patients enrolled in the CLIMB (Comprehensive Longitudinal Investigation of Multiple Sclerosis at Brigham and Women's Hospital) study and biorepository who had been vaccinated against COVID-19 and had SARS-CoV-2 spike antibody (anti-SARS-CoV-2 S Roche-Elecsys) testing were identified and compared to healthy controls. Demographic data, serum immune profiles including lymphocyte count, B-cell count, and immunoglobulins, and clinical outcome of COVID-19 infection were collected.
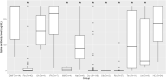
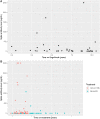
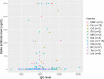
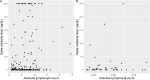
Results: 254 patients (73.2% female, mean (SD) age 52.9 (11.2) years) were identified. When controlling for age, time since vaccination, and vaccine type, patients on fingolimod, ocrelizumab, rituximab, mycophenolate mofetil, natalizumab and teriflunomide had significantly lower levels of spike antibodies compared to healthy controls (n = 34). Longer duration of treatment was associated with lower spike antibody levels in patients on anti-CD20 therapy (p = 0.016) and S1P modulators (p = 0.016) compared to healthy controls. In patients on anti-CD20 therapy, higher spike antibody levels were associated with higher CD20 cell count (p<0.001), and longer time since last anti-CD20 therapy infusion (p<0.001). 92.8% (13/14) vaccine responders (spike antibody titer >100 ug/dL) on anti-CD20 therapy demonstrated B-cell reconstitution (mean CD20 3.6%). Only 1 out of 86 patients with CD20 of 0% had a measurable spike antibody response to vaccination. During follow-up (mean 270 days), five patients were diagnosed with COVID-19 after vaccination (incidence 1.9%), all of whom had spike antibody < 20 ug/dL. No patients required ICU care or died.
Conclusions: Patients on some DMTs demonstrate reduced humoral immunity after Sars-CoV-2 vaccination. Longer duration of anti-CD20 therapy and reduced CD20 cell count is associated with blunted humoral response to vaccination. CD20 reconstitution >0.1% appears necessary, but not always sufficient, for humoral response to vaccination. Breakthrough COVID-19 infection in our cohort of MS patients on DMT was higher than in population studies. We propose that adjustment of B-cell therapy administration to allow for B-cell reconstitution prior to vaccination should be considered.
Keywords: Anti-CD20; COVID-19; Disease modifying therapy; Multiple sclerosis; Vaccination.
Copyright © 2022. Published by Elsevier B.V.
Figures
Similar articles
-
Longitudinal adaptive immune responses following sequential SARS-CoV-2 vaccinations in MS patients on anti-CD20 therapies and sphingosine-1-phosphate receptor modulators.Mult Scler Relat Disord. 2023 Feb;70:104484. doi: 10.1016/j.msard.2022.104484. Epub 2022 Dec 28. Mult Scler Relat Disord. 2023. PMID: 36608538 Free PMC article.
-
Post-vaccination SARS-Cov-2 T-cell receptor repertoires in patients with multiple sclerosis and related disorders.Mult Scler Relat Disord. 2023 Nov;79:104965. doi: 10.1016/j.msard.2023.104965. Epub 2023 Aug 28. Mult Scler Relat Disord. 2023. PMID: 37657307
-
Antibody response to SARS-CoV-2 vaccination following typical and three-dose dosing schedules in multiple sclerosis patients treated with disease modifying therapies.Mult Scler Relat Disord. 2022 Jul;63:103856. doi: 10.1016/j.msard.2022.103856. Epub 2022 May 6. Mult Scler Relat Disord. 2022. PMID: 35636275 Free PMC article.
-
Risk of COVID-19 infection and severe disease in MS patients on different disease-modifying therapies.Mult Scler Relat Disord. 2022 Apr;60:103735. doi: 10.1016/j.msard.2022.103735. Epub 2022 Mar 11. Mult Scler Relat Disord. 2022. PMID: 35398713 Free PMC article.
-
Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: a systematic review/meta-analysis.Ann Clin Transl Neurol. 2022 Aug;9(8):1321-1331. doi: 10.1002/acn3.51628. Epub 2022 Jul 19. Ann Clin Transl Neurol. 2022. PMID: 35852423 Free PMC article.
Cited by
-
Immune monitoring of SARS-CoV-2-specific T cell and B cell responses in patients with multiple sclerosis treated with ocrelizumab.Front Immunol. 2023 Sep 20;14:1254128. doi: 10.3389/fimmu.2023.1254128. eCollection 2023. Front Immunol. 2023. PMID: 37841269 Free PMC article.
-
Effects of vaccination on COVID-19 infection symptoms in multiple sclerosis patients.eNeurologicalSci. 2024 Jun 12;36:100511. doi: 10.1016/j.ensci.2024.100511. eCollection 2024 Sep. eNeurologicalSci. 2024. PMID: 38989276 Free PMC article.
-
Anti-RBD Antibody Levels and IFN-γ-Specific T Cell Response Are Associated with a More Rapid Swab Reversion in Patients with Multiple Sclerosis after the Booster Dose of COVID-19 Vaccination.Vaccines (Basel). 2024 Aug 19;12(8):926. doi: 10.3390/vaccines12080926. Vaccines (Basel). 2024. PMID: 39204049 Free PMC article.
-
Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells.Cells. 2025 Apr 17;14(8):606. doi: 10.3390/cells14080606. Cells. 2025. PMID: 40277931 Free PMC article. Review.
-
Immunologic and Autoimmune-Related Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Clinical Symptoms and Mechanisms of Disease.Phys Med Rehabil Clin N Am. 2023 Aug;34(3):623-642. doi: 10.1016/j.pmr.2023.04.004. Epub 2023 Apr 11. Phys Med Rehabil Clin N Am. 2023. PMID: 37419536 Free PMC article. Review.
References
-
- Cohen JA, Bermel RA, Grossman CI, et al. Immunoglobulin G immune response to SARS-CoV-2 vaccination in people living with multiple sclerosis within multiple sclerosis partners advancing technology and health solutions. Mult. Scler. 2022 doi: 10.1177/13524585211061343. Jan 0713524585211061343. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

