Refining the definition of the avian pathogenic Escherichia coli (APEC) pathotype through inclusion of high-risk clonal groups
- PMID: 35952599
- PMCID: PMC9385700
- DOI: 10.1016/j.psj.2022.102009
Refining the definition of the avian pathogenic Escherichia coli (APEC) pathotype through inclusion of high-risk clonal groups
Abstract
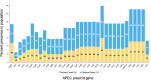
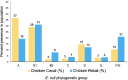
Colibacillosis in poultry is a unique disease manifestation of Escherichia coli in the animal world, as one of the primary routes of entry is via the respiratory tract of birds. Because of this, a novel extraintestinal pathogenic E. coli (ExPEC) subpathotype coined avian pathogenic E. coli (or APEC) has been described. Like other ExPEC, this pathotype has been challenging to clearly define, and in the case of APEC, its role as an opportunistic pathogen has further complicated these challenges. Using 3,479 temporally matched genomes of poultry-source isolates, we show that the APEC plasmid, previously considered a defining trait of APEC, is highly prevalent in clinical isolates from diseased turkeys. However, the plasmid is also quite prevalent among cecal E. coli isolates from healthy birds, including both turkeys and broilers. In contrast, we identify distinct differences in clonal backgrounds of turkey clinical versus cecal strains, with a subset of sequence types (STs) dominating the clinical landscape (ST23, ST117, ST131, ST355, and ST428), which are rare within the cecal landscape. Because the same clinical STs have also dominated the broiler landscape, we performed lethality assays using strains from dominant STs from clinical or cecal landscapes in embryonated turkey and chicken eggs. We show that, irrespective of plasmid carriage, dominant clinical STs are significantly more virulent than dominant cecal STs. We present a revised APEC screening tool that incorporates APEC plasmid carriage plus markers for dominant clinical STs. This revised APEC pathotyping tool improves the ability to identify high-risk APEC clones within poultry production systems, and identifies STs of interest for mitigation targets.
Keywords: APEC; Escherichia coli; colibacillosis; pathotype; poultry.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. - PubMed
-
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., Pyshkin A.V., Sirotkin A.V., Vyahhi N., Tesler G., Alekseyev M.A., Pevzner P.A. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

