Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape
- PMID: 35952703
- PMCID: PMC9896921
- DOI: 10.1016/S1473-3099(22)00291-2
Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape
Abstract
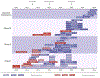
Respiratory syncytial virus is the second most common cause of infant mortality and a major cause of morbidity and mortality in older adults (aged >60 years). Efforts to develop a respiratory syncytial virus vaccine or immunoprophylaxis remain highly active. 33 respiratory syncytial virus prevention candidates are in clinical development using six different approaches: recombinant vector, subunit, particle-based, live attenuated, chimeric, and nucleic acid vaccines; and monoclonal antibodies. Nine candidates are in phase 3 clinical trials. Understanding the epitopes targeted by highly neutralising antibodies has resulted in a shift from empirical to rational and structure-based vaccine and monoclonal antibody design. An extended half-life monoclonal antibody for all infants is likely to be within 1 year of regulatory approval (from August, 2022) for high-income countries. Live-attenuated vaccines are in development for older infants (aged >6 months). Subunit vaccines are in late-stage trials for pregnant women to protect infants, whereas vector, subunit, and nucleic acid approaches are being developed for older adults. Urgent next steps include ensuring access and affordability of a respiratory syncytial virus vaccine globally. This review gives an overview of respiratory syncytial virus vaccines and monoclonal antibodies in clinical development highlighting different target populations, antigens, and trial results.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests UMCU received minor funding from The Bill & Melinda Gates Medical Research Institute. DMW has received consulting fees from Pfizer, Merck, Affinivax, and Matrivax, unrelated to this manuscript; and is a principal investigator with grant support from Pfizer and Merck to Yale University, unrelated to this manuscript. NIM has regular interaction with pharameutical and other industrial partners: UMC Utrecht has received fees for invited lectures by Abbvie, Merck, and Sanofi. LB has regular interaction with pharmaceutical and other industrial partners. BSG is an inventor of patents for RSV vaccines using the stabilised prefusion F protein. AM has received research grants from National Institutes of Health, Janssen, and Merck institution; fees for participation in advisory boards from Janssen, Sanofi-Pasteur, and Merck; and fees for educational lectures from Sanofi-Pasteur and AstraZeneca. TJR was financially supported by the Intramural Program of the National Institute of Allergy & Infectious Diseases, National Institute of Health. BSG was financially supported by the Intramural Program of the National Institute of Allergy & Infectious Diseases, National Institute of Health. UJB was financially supported by the Intramural Program of the National Institute of Allergy & Infectious Diseases, National Institute of Health. UMCU has received major funding (>€100 000 per industrial partner) for investigator-initiated studies from AbbVie, MedImmune, Janssen, Pfizer, the Bill & Melinda Gates Foundation, and MeMed Diagnostics. UMCU has received major cash or in-kind funding as part of the public private partnership IMI-funded RESCEU project from GSK, Novavax, Janssen, AstraZeneca, Pfizer, and Sanofi. UMCU has received major funding by Julius Clinical for participating in the INFORM study sponsored by MedImmune. UMCU has received minor funding for participation in trials by Regeneron and Janssen from 2015-17 (total annual estimate less than €20 000). UMCU received minor funding for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, Novavax, Pfizer, and Janssen (total annual estimate less than €20 000). Dr. Bont is the founding chairman of the ReSViNET Foundation.
Figures



References
-
- Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378: 1917–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

