Viral load may impact the diagnostic performance of nasal swabs in nucleic acid amplification test and quantitative antigen test for SARS-CoV-2 detection
- PMID: 35953013
- PMCID: PMC9359764
- DOI: 10.1016/j.jiac.2022.07.023
Viral load may impact the diagnostic performance of nasal swabs in nucleic acid amplification test and quantitative antigen test for SARS-CoV-2 detection
Abstract
Introduction: Compared to nasopharyngeal swabs (NPS), there has been insufficient evaluation of the diagnostic performance of nasal swabs (NS) for the detection of severe acute respiratory coronavirus 2 (SARS-CoV-2) in the nucleic acid amplification test (NAAT) and quantitative SARS-CoV-2 antigen test (QAT).
Methods: We prospectively compared healthcare worker-collected and flocked NS within nine days after symptom onset to paired NPS to detect SARS-CoV-2 in NAAT and QAT on the fully automated Lumipulse system. The agreement between sample types was evaluated, and cycle threshold (Ct) values and antigen levels were used as surrogate viral load measures.
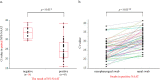
Results: Sixty sets of NPS and NS samples were collected from 40 patients with COVID-19. The overall agreements between NAAT and QAT samples were 76.7% and 65.0%, respectively. In NAAT, the Ct value of NS was significantly higher, 5.9, than that of NPS. Thirty-nine (95.1%) NS tested positive in 41 positive-paired NPS with Ct ≤ 30. The negative correlation was observed between antigen levels of NS in QAT and Ct values of NS in NAAT (r = -0.88). In QAT, the antigen level of NS was significantly lower than that of NPS. Thirty-six (90.0%) NS tested positive in 40 positive-paired NPS with antigen levels >100 pg/mL, which were collected significantly earlier than those with antigen levels ≤100 pg/mL.
Conclusions: In NAAT and QAT, NS had limited performance in detecting SARS-CoV-2 compared to NPS. However, NS may be helpful for patients with COVID-19 with high viral loads or those in the early stages of the illness.
Keywords: Nasal swabs; Nasopharyngeal swabs; Nucleic acid amplification test; Quantitative SARS-CoV-2 antigen test; SARS-CoV-2; Viral load.
Copyright © 2022 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest Satoshi Takahashi received speaker honoraria from MSD K.K. and research grants from Shino-Test Corporation and Abbott Japan Co., Ltd.
Figures

References
-
- World Health Organization Diagnostic testing for SARS-CoV-2 interim guidance 11 september 2020. https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-...
-
- Hanson K.E., Barker A.P., Hillyard D.R., Gilmore N., Barrett J.W., Orlandi R.R., et al. Self-collected anterior nasal and saliva specimens versus healthcare worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01824-20. 20. - DOI - PMC - PubMed
-
- Callahan C., Lee R., Lee G., Zulauf K.E., Kirby J.E., Arnaout R. Nasal-swab testing misses patients with low SARS-CoV-2 viral loads. medRxiv. 2020 doi: 10.1101/2020.06.12.20128736. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

