Clinical Characteristics and Transplant-Free Survival Across the Spectrum of Pulmonary Vascular Disease
- PMID: 35953136
- PMCID: PMC9897285
- DOI: 10.1016/j.jacc.2022.05.038
Clinical Characteristics and Transplant-Free Survival Across the Spectrum of Pulmonary Vascular Disease
Abstract
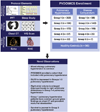
Background: PVDOMICS (Pulmonary Vascular Disease Phenomics) is a precision medicine initiative to characterize pulmonary vascular disease (PVD) using deep phenotyping. PVDOMICS tests the hypothesis that integration of clinical metrics with omic measures will enhance understanding of PVD and facilitate an updated PVD classification.
Objectives: The purpose of this study was to describe clinical characteristics and transplant-free survival in the PVDOMICS cohort.
Methods: Subjects with World Symposium Pulmonary Hypertension (WSPH) group 1-5 PH, disease comparators with similar underlying diseases and mild or no PH and healthy control subjects enrolled in a cross-sectional study. PH groups, comparators were compared using standard statistical tests including log-rank tests for comparing time to transplant or death.
Results: A total of 1,193 subjects were included. Multiple WSPH groups were identified in 38.9% of PH subjects. Nocturnal desaturation was more frequently observed in groups 1, 3, and 4 PH vs comparators. A total of 50.2% of group 1 PH subjects had ground glass opacities on chest computed tomography. Diffusing capacity for carbon monoxide was significantly lower in groups 1-3 PH than their respective comparators. Right atrial volume index was higher in WSPH groups 1-4 than comparators. A total of 110 participants had a mean pulmonary artery pressure of 21-24 mm Hg. Transplant-free survival was poorest in group 3 PH.
Conclusions: PVDOMICS enrolled subjects across the spectrum of PVD, including mild and mixed etiology PH. Novel findings include low diffusing capacity for carbon monoxide and enlarged right atrial volume index as shared features of groups 1-3 and 1-4 PH, respectively; unexpected, frequent presence of ground glass opacities on computed tomography; and sleep alterations in group 1 PH, and poorest survival in group 3 PH. PVDOMICS will facilitate a new understanding of PVD and refine the current PVD classification. (Pulmonary Vascular Disease Phenomics Program PVDOMICS [PVDOMICS]; NCT02980887).
Keywords: phenotyping; pulmonary hypertension.
Copyright © 2022 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures The study received grants U01 HL125218 (Principal Investigator: Dr Rosenzweig), U01 HL125205 (Principal Investigator: Dr Frantz), U01 HL125212 (Principal Investigator: Dr Hemnes), U01 HL125208 (Principal Investigator: Dr Rischard), U01 HL125175 (Principal Investigator: Dr Hassoun), U01 HL125215 (Principal Investigator: Dr Leopold), and U01 HL125177 (Principal Investigator: Dr Beck) and was supported by the Pulmonary Hypertension Association. Dr Hemnes has served as a consultant for Bayer, United Therapeutics, Janssen, GossamerBio, and Tenax Therapeutics; holds stock in Tenax Therapeutics; and has received grants from the National Institutes of Health, CMREF, and Imara. Dr Leopold is supported by the National Institutes of Health grant U01 125215 and the American Heart Association 19AIML34980000; receives salary support from the Massachusetts Medical Society; has received research funding (to her institution) from Astellas; has served as a consultant for Abbott Vascular and United Therapeutics; and has served as a site principal investigator for a study sponsored by Aria CV. Dr Abidov is supported by research grants from Astellas Pharma, Boehringer Ingelheim, and Kiniksa outside of the submitted work. Dr Rosenzweig has received consulting fees from Acceleron for a scientific advisory board meeting; and her institution receives grant support from Bayer, United Therapeutics, Janssen, and SonVie. Dr Borlaug has received research grants from National Institutes of Health/NHLBI, AstraZeneca, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and has received consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr Dubrock has received consulting fees from Janssen Pharmaceuticals; and has served on advisory boards for Janssen Pharmaceuticals and United Therapeutics. Dr Finet is a consultant to Wolters Kluwer Health, Clinical Drug Information Ad Honorem. Dr Frantz has consulting, steering committee, and advisory board relationships with Altavant Sciences, Bayer, Gossamer Bio, Janssen, Shouti, France Foundation, IQVIA, Tenax, UpToDate, and United Therapeutics. Dr Garcia is CEO and founder of Aqualing Therapeutics. Dr Hassoun has served as scientific advisor for Merck Sharp & Dohme, an activity unrelated to the current work. Dr Highland has grants/contracts with Acceleron Pharmaceuticals, Actelion Pharmaceuticals (Janssen), Bayer Healthcare, Boehringer Ingelheim, Eiger Pharmaceuticals, Eli Lilly, Gossamer Bio, United Therapeutics, and Viela Bio (Horizon); has served as a consultant and/or member of a steering or advisory committee with Acceleron Pharmaceuticals, Actelion Pharmaceuticals (Janssen), Boehringer Ingelheim, Forsee, Genentech, Gossamer Bio, and United Therapeutics; and has served on the Speakers Bureau for Actelion Pharmaceuticals (Janssen), Bayer Healthcare, Boehringer Ingelheim, and United Therapeutics. Dr Hill has received research grants for Acceleron, Aerovate. Altavant, Gossamer. Liquidia, Merck, and United Therapeutics; and has served on advisory boards for Acceleron, Aerovate, Altavant, Gossamer, and Liquidia. Dr Maron has relationships with Deerfield Corporation, Actelion Sciences, and Tenax Therapeutics; and has U.S. Patent #9,605,047, Patent pending PCT/US2019/059890, Patent application 2021/133937. Dr Mathai has served as a consultant for Acceleron, Actelion, Bayer, and United Therapeutics. Dr Mehra is supported by National Institutes of Health grants U01HL125177 and UH3HL140144 and the American Heart Association AHA 18SFRN34170013; has received royalties from Up to Date; has received compensation from the American Board of Internal Medicine; and has received an honorarium from the American Academy of Sleep Medicine. M.M. Park has served on the Speakers Bureau of Lantheus Medical Imaging (Definity contrast). Dr Rischard has consulting relationships with Acceleron and United Therapeutics; is on a Steering Committee for Acceleron; and receives research support from Ismed, United Therapeutics, Bayer, Acceleron, Janssen, and AADI. Dr Thomas has served as a consultant for GE, Abbott, egnite, EchoIQ, and Caption Health; as well as spouse employment for Caption Health. Dr Horn has served on the Data and Safety Monitoring Board of AADi Biosciences and SoniVie; has served on the Clinical Events Committee for V-wave; and has served as a consultant for Biotronik. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






Comment in
-
Pulmonary Hypertension: Dissecting a Complex Phenotype.J Am Coll Cardiol. 2022 Aug 16;80(7):719-721. doi: 10.1016/j.jacc.2022.05.039. J Am Coll Cardiol. 2022. PMID: 35953137 No abstract available.
References
-
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HL141387/HL/NHLBI NIH HHS/United States
- U01 HL125218/HL/NHLBI NIH HHS/United States
- R35 HL140019/HL/NHLBI NIH HHS/United States
- R01 HL163960/HL/NHLBI NIH HHS/United States
- U01 HL125205/HL/NHLBI NIH HHS/United States
- U01 HL125177/HL/NHLBI NIH HHS/United States
- R01 HL158746/HL/NHLBI NIH HHS/United States
- U01 HL125215/HL/NHLBI NIH HHS/United States
- U01 HL125208/HL/NHLBI NIH HHS/United States
- R01 HL142720/HL/NHLBI NIH HHS/United States
- R01 HL136603/HL/NHLBI NIH HHS/United States
- UH3 HL140144/HL/NHLBI NIH HHS/United States
- U01 HL125175/HL/NHLBI NIH HHS/United States
- R01 HL133721/HL/NHLBI NIH HHS/United States
- U01 HL125212/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

