Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice
- PMID: 35953274
- PMCID: PMC9451628
- DOI: 10.3174/ajnr.A7586
Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice
Abstract
Monoclonal antibodies are emerging disease-modifying therapies for Alzheimer disease that require brain MR imaging for eligibility assessment as well as for monitoring for amyloid-related imaging abnormalities. Amyloid-related imaging abnormalities result from treatment-related loss of vascular integrity and may occur in 2 forms. Amyloid-related imaging abnormalities with edema or effusion are transient, treatment-induced edema or sulcal effusion, identified on T2-FLAIR. Amyloid-related imaging abnormalities with hemorrhage are treatment-induced microhemorrhages or superficial siderosis identified on T2* gradient recalled-echo. As monoclonal antibodies become more widely available, treatment screening and monitoring brain MR imaging examinations may greatly increase neuroradiology practice volumes. Radiologists must become familiar with the imaging appearance of amyloid-related imaging abnormalities, how to select an appropriate imaging protocol, and report findings in clinical practice. On the basis of clinical trial literature and expert experience from clinical trial imaging, we summarize imaging findings of amyloid-related imaging abnormalities, describe potential interpretation pitfalls, and provide recommendations for a standardized imaging protocol and an amyloid-related imaging abnormalities reporting template. Standardized imaging and reporting of these findings are important because an amyloid-related imaging abnormalities severity score, derived from the imaging findings, is used along with clinical status to determine patient management and eligibility for continued monoclonal antibody dosing.
© 2022 by American Journal of Neuroradiology.
Figures
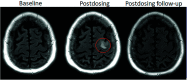
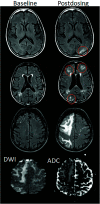
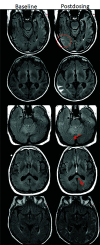
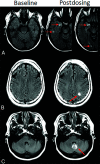
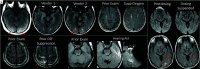
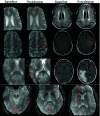
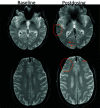
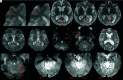

Comment in
-
MRI Monitoring of Anti-Alzheimer Therapy Amyloid-Related Imaging Abnormalities: Due Diligence or Overkill?AJNR Am J Neuroradiol. 2023 Jan;44(1):E4-E5. doi: 10.3174/ajnr.A7702. Epub 2022 Dec 8. AJNR Am J Neuroradiol. 2023. PMID: 36574317 Free PMC article. No abstract available.
-
CAA-ri and ARIA: Two Faces of the Same Coin?AJNR Am J Neuroradiol. 2023 Feb;44(2):E13-E14. doi: 10.3174/ajnr.A7759. Epub 2023 Jan 12. AJNR Am J Neuroradiol. 2023. PMID: 36635054 Free PMC article. No abstract available.
References
-
- ADUHELM®. (aducanumab-avwa) is FDA Approved for Healthcare Professionals. https://biogencdn.com/us/aduhelm-pi.pdf. Accessed June 7, 2021
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
