Crossed Corticostriatal Projections in the Macaque Brain
- PMID: 35953294
- PMCID: PMC9480880
- DOI: 10.1523/JNEUROSCI.0071-22.2022
Crossed Corticostriatal Projections in the Macaque Brain
Abstract
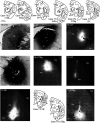
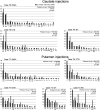
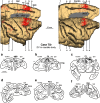
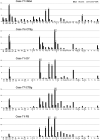
In nonhuman primates, major input to the striatum originates from ipsilateral cortex and thalamus. The striatum is a target also of crossed corticostriatal (CSt) projections from the contralateral hemisphere, which have been so far somewhat neglected. In the present study, based on neural tracer injections in different parts of the striatum in macaques of either sex, we analyzed and compared qualitatively and quantitatively the distribution of labeled CSt cells in the two hemispheres. The results showed that crossed CSt projections to the caudate and the putamen can be relatively robust (up to 30% of total labeled cells). The origin of the direct and the crossed CSt projections was not symmetrical as the crossed ones originated almost exclusively from motor, prefrontal, and cingulate areas and not from parietal and temporal areas. Furthermore, there were several cases in which the contribution of contralateral areas tended to equal that of the ipsilateral ones. The present study is the first detailed description of this anatomic pathway of the macaque brain and provides the substrate for bilateral distribution of motor, motivational, and cognitive signals for reinforcement learning and selection of actions or action sequences, and for learning compensatory motor strategies after cortical stroke.SIGNIFICANCE STATEMENT In nonhuman primates the striatum is a target of projections originating from the contralateral hemisphere (crossed CSt projections), which have been so far poorly investigated. The present study analyzed qualitatively and quantitatively in the macaque brain the origin of the crossed CSt projections compared with those originating from the ipsilateral hemisphere. The results showed that crossed CSt projections originate mostly from frontal and rostral cingulate areas and in some cases their contribution tended to equal that from ipsilateral areas. These projections could provide the substrate for bilateral distribution of motor, motivational, and cognitive signals for reinforcement learning and action selection, and for learning compensatory motor strategies after cortical stroke.
Keywords: basal ganglia; cingulate cortex; frontal cortex; interhemispheric transfer; monkey; striatum.
Copyright © 2022 the authors.
Figures




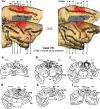


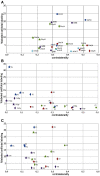
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
