Investigating Tau and Amyloid Tracer Skull Binding in Studies of Alzheimer Disease
- PMID: 35953305
- PMCID: PMC9902848
- DOI: 10.2967/jnumed.122.263948
Investigating Tau and Amyloid Tracer Skull Binding in Studies of Alzheimer Disease
Abstract
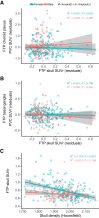
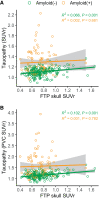
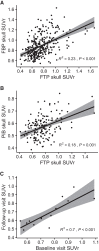
Off-target binding of [18F]flortaucipir (FTP) can complicate quantitative PET analyses. An underdiscussed off-target region is the skull. Here, we characterize how often FTP skull binding occurs, its influence on estimates of Alzheimer disease pathology, its potential drivers, and whether skull uptake is a stable feature across time and tracers. Methods: In 313 cognitively normal and mildly impaired participants, CT scans were used to define a skull mask. This mask was used to quantify FTP skull uptake. Skull uptake of the amyloid-β PET tracers [18F]florbetapir and [11C]Pittsburgh compound B (n = 152) was also assessed. Gaussian mixture modeling defined abnormal levels of skull binding for each tracer. We examined the relationship of continuous bone uptake to known off-target binding in the basal ganglia and choroid plexus as well as skull density measured from the CT. Finally, we examined the confounding effect of skull binding on pathologic quantification. Results: We found that 50 of 313 (∼16%) FTP scans had high levels of skull signal. Most were female (n = 41, 82%), and in women, lower skull density was related to higher FTP skull signal. Visual reads by a neuroradiologist revealed a significant relationship with hyperostosis; however, only 21% of women with high skull binding were diagnosed with hyperostosis. FTP skull signal did not substantially correlate with other known off-target regions. Skull uptake was consistent over longitudinal FTP scans and across tracers. In amyloid-β-negative, but not -positive, individuals, FTP skull binding impacted quantitative estimates in temporal regions. Conclusion: FTP skull binding is a stable, participant-specific phenomenon and is unrelated to known off-target regions. Effects were found primarily in women and were partially related to lower bone density. The presence of [11C]Pittsburgh compound B skull binding suggests that defluorination does not fully explain FTP skull signal. As signal in skull bone can impact quantitative analyses and differs across sex, it should be explicitly addressed in studies of aging and Alzheimer disease.
Keywords: amyloid PET; human; off-target binding; tau PET.
© 2023 by the Society of Nuclear Medicine and Molecular Imaging.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG066444/AG/NIA NIH HHS/United States
- K01 AG053474/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- T32 AG058518/AG/NIA NIH HHS/United States
- RF1 AG073424/AG/NIA NIH HHS/United States
- R01 EB009352/EB/NIBIB NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P30 NS098577/NS/NINDS NIH HHS/United States
- S10 OD025214/OD/NIH HHS/United States
- R01 AG055444/AG/NIA NIH HHS/United States
- U01 AG042791/AG/NIA NIH HHS/United States
- R01 AG031581/AG/NIA NIH HHS/United States
- R01 AG046179/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
