Seasonal influenza vaccine uptake among patients with cardiovascular disease in Denmark, 2017-2019
- PMID: 35953403
- PMCID: PMC10405130
- DOI: 10.1093/ehjqcco/qcac049
Seasonal influenza vaccine uptake among patients with cardiovascular disease in Denmark, 2017-2019
Abstract
Background: Influenza vaccination protects against morbidity and mortality in patients with cardiovascular disease (CVD). We aimed to describe influenza vaccine uptake in patients with CVD in a universal-access healthcare system.
Methods: Using nationwide Danish registries, we included all patients with prevalent CVD, defined as heart failure (HF), atrial fibrillation (AF), ischemic heart disease (IHD), or stroke during three consecutive influenza seasons (October-December 2017-2019). The outcome was relative frequency of influenza vaccination across strata of patient characteristics.
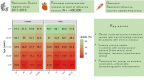
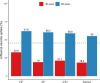
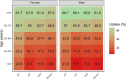
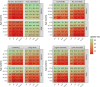
Results: There was an average of 397 346 patients with CVD yearly during 2017-2019. Vaccine uptake was 45.6% for the whole population and ranged from 55.0% in AF to 61.8% in HF among patients aged ≥65 years. Among patients aged <65 years, uptake was 32.6% in HF, 19.0% in AF, 21.1% in IHD, and 18.3% in stroke. There was a lower uptake with decreasing age: 21.6% in HF, 5.5% in AF, 7.4% in IHD, and 6.3% in stroke among males aged <45 years, as opposed to 25.5% in HF, 11.5% in AF, 13.8% in IHD, and 12.1% in stroke for males aged 45-54 years. In the further stratified analyses, uptake ranged from a low of 2.5% for males <45 years with AF who were not vaccinated the previous season to a high of 87.0% for females ≥75 years with IHD who were vaccinated the previous season.
Conclusion: Seasonal influenza vaccine uptake is suboptimal among patients with CVD, even in a universal-access healthcare system with free-of-charge vaccinations. Vaccine uptake was particularly low among young patients.
Keywords: Cardiovascular; Influenza; Registry; Vaccine.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Dr Tor Biering-Sørensen reports the following: Steering Committee member of the Amgen financed GALACTIC-HF trial; Chief investigator and steering committee chair of the Sanofi Pasteur financed ‘NUDGE-FLU’ trial; Chief investigator and steering committee chair of the Sanofi Pasteur financed ‘DANFLU-1’ trial; Chief investigator and steering committee chair of the Sanofi Pasteur financed ‘DANFLU-2’ trial; Steering Committee member of ‘LUX-Dx TRENDS Evaluates Diagnostics Sensors in Heart Failure Patients Receiving Boston Scientific's Investigational ICM System’ trial; Advisory Board: Sanofi Pasteur, Amgen and GSK; Speaker Honorarium: Novartis, Sanofi Pasteur and GSK; Research grants: GE Healthcare and Sanofi Pasteur. Dr Christian Torp-Pedersen reports the following: Grants for studies from Bayer and Novo Nordisk unrelated to the current study. Dr Schou reports lecture fees from Novo Nordisk, Astra Zeneca, Novartis, and Boehringer Ingelheim outside the current study. All other authors had no conflicts of interest to disclose.
Figures
References
-
- Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow Tet al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018;378:345–353. - PubMed
-
- Ciszewski A, Bilinska ZT, Brydak LB, Kepka C, Kruk M, Romanowska Met al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J 2008;29:1350–1358. - PubMed
-
- Modin D, Jørgensen ME, Gislason G, Jensen JS, Køber L, Claggett Bet al. Influenza vaccine in heart failure. Circulation 2019;139:575–586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

