Mapping astrogliosis in the individual human brain using multidimensional MRI
- PMID: 35953450
- PMCID: PMC9976979
- DOI: 10.1093/brain/awac298
Mapping astrogliosis in the individual human brain using multidimensional MRI
Abstract
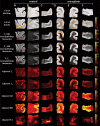
There are currently no non-invasive imaging methods available for astrogliosis assessment or mapping in the central nervous system despite its essential role in the response to many disease states, such as infarcts, neurodegenerative conditions, traumatic brain injury and infection. Multidimensional MRI is an increasingly employed imaging modality that maximizes the amount of encoded chemical and microstructural information by probing relaxation (T1 and T2) and diffusion mechanisms simultaneously. Here, we harness the exquisite sensitivity of this imagining modality to derive a signature of astrogliosis and disentangle it from normative brain at the individual level using machine learning. We investigated ex vivo cerebral cortical tissue specimens derived from seven subjects who sustained blast-induced injuries, which resulted in scar-border forming astrogliosis without being accompanied by other types of neuropathological abnormality, and from seven control brain donors. By performing a combined post-mortem radiology and histopathology correlation study we found that astrogliosis induces microstructural and chemical changes that are robustly detected with multidimensional MRI, and which can be attributed to astrogliosis because no axonal damage, demyelination or tauopathy were histologically observed in any of the cases in the study. Importantly, we showed that no one-dimensional T1, T2 or diffusion MRI measurement can disentangle the microscopic alterations caused by this neuropathology. Based on these findings, we developed a within-subject anomaly detection procedure that generates MRI-based astrogliosis biomarker maps ex vivo, which were significantly and strongly correlated with co-registered histological images of increased glial fibrillary acidic protein deposition (r = 0.856, P < 0.0001; r = 0.789, P < 0.0001; r = 0.793, P < 0.0001, for diffusion-T2, diffusion-T1 and T1-T2 multidimensional data sets, respectively). Our findings elucidate the underpinning of MRI signal response from astrogliosis, and the demonstrated high spatial sensitivity and specificity in detecting reactive astrocytes at the individual level, and if reproduced in vivo, will significantly impact neuroimaging studies of injury, disease, repair and aging, in which astrogliosis has so far been an invisible process radiologically.
Keywords: GFAP; astrogliosis; blast; diffusion; machine learning; multidimensional MRI; radiological–pathological correlations; relaxation; traumatic brain injury.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures






Comment in
-
Multidimensional MRI detects astrogliosis.Nat Rev Neurol. 2022 Oct;18(10):575. doi: 10.1038/s41582-022-00717-7. Nat Rev Neurol. 2022. PMID: 36071268 No abstract available.
References
-
- Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. - PubMed
-
- Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol Rev. 2014;94:1077–1098. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

