Biocatalytic routes to stereo-divergent iridoids
- PMID: 35953485
- PMCID: PMC9372074
- DOI: 10.1038/s41467-022-32414-w
Biocatalytic routes to stereo-divergent iridoids
Erratum in
-
Author Correction: Biocatalytic routes to stereo-divergent iridoids.Nat Commun. 2022 Sep 27;13(1):5678. doi: 10.1038/s41467-022-33380-z. Nat Commun. 2022. PMID: 36167838 Free PMC article. No abstract available.
Abstract
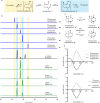
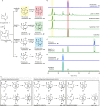
Thousands of natural products are derived from the fused cyclopentane-pyran molecular scaffold nepetalactol. These natural products are used in an enormous range of applications that span the agricultural and medical industries. For example, nepetalactone, the oxidized derivative of nepetalactol, is known for its cat attractant properties as well as potential as an insect repellent. Most of these naturally occurring nepetalactol-derived compounds arise from only two out of the eight possible stereoisomers, 7S-cis-trans and 7R-cis-cis nepetalactols. Here we use a combination of naturally occurring and engineered enzymes to produce seven of the eight possible nepetalactol or nepetalactone stereoisomers. These enzymes open the possibilities for biocatalytic production of a broader range of iridoids, providing a versatile system for the diversification of this important natural product scaffold.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Fang, C., Fernie, A. R. & Luo, J. Exploring the diversity of plant metabolism. Trends Plant Sci. 10.1016/j.tplants.2018.09.006 (2019). - PubMed
-
- Weng JK. The evolutionary paths towards complexity: a metabolic perspective. N. Phytol. 2014;201:1141–1149. - PubMed
-
- Maeda, H. A. & Fernie, A. R. Evolutionary history of plant metabolism. Ann. Rev. Plant Biol.10.1146/annurev-arplant-080620-031054 (2021). - PubMed
-
- Dinda B, Debnath S, Harigaya Y. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull. 2007;55:159–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

