Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or mRNA-1273-boosting with homologous COVID-19 vaccine regimens
- PMID: 35953492
- PMCID: PMC9366133
- DOI: 10.1038/s41467-022-32321-0
Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or mRNA-1273-boosting with homologous COVID-19 vaccine regimens
Abstract
Comparative analyses of the immunogenicity and reactogenicity of homologous and heterologous SARS-CoV-2 vaccine-regimens will inform optimized vaccine strategies. Here we analyze the humoral and cellular immune response following heterologous and homologous vaccination strategies in a convenience cohort of 331 healthy individuals. All regimens induce immunity to the vaccine antigen. Immunity after vaccination with ChAdOx1-nCoV-19 followed by either BNT162b2 (n = 66) or mRNA-1273 (n = 101) is equivalent to or more pronounced than homologous mRNA-regimens (n = 43 BNT162b2, n = 59 mRNA-1273) or homologous ChAdOx1-nCoV-19 vaccination (n = 62). We note highest levels of spike-specific CD8 T-cells following both heterologous regimens. Among mRNA-containing combinations, spike-specific CD4 T-cell levels in regimens including mRNA-1273 are higher than respective combinations with BNT162b2. Polyfunctional T-cell levels are highest in regimens based on ChAdOx1-nCoV-19-priming. All five regimens are well tolerated with most pronounced reactogenicity upon ChAdOx1-nCoV-19-priming, and ChAdOx1-nCoV-19/mRNA-1273-boosting. In conclusion, we present comparative analyses of immunogenicity and reactogenicity for heterologous vector/mRNA-boosting and homologous mRNA-regimens.
© 2022. The Author(s).
Conflict of interest statement
M.S. has received grant support from Astellas and Biotest to the organization Saarland University outside the submitted work, and honoraria for lectures from Biotest and Novartis. All other authors of this manuscript have no conflicts of interest to disclose.
Figures
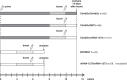



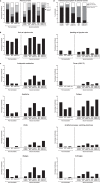
References
-
- Vygen-Bonnet S, et al. Beschluss der STIKO zur 5. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. Epid. Bull. 2021;19:24–36.
-
- Koch J, et al. STIKO-Empfehlung zur COVID-19-Auffrischimpfung mit einem mRNA Impfstoff für Personen ≥70 Jahre und bestimmte Indikationsgruppen sowie Empfehlung zur Optimierung der Grundimmunisierung mit einem mRNA Impfstoff nach vorausgegangener Impfung mit der COVID-19 Vaccine Janssen und die dazugehörige wissenschaftliche Begründung. Epid. Bull. 2021;43:16–53.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

