Discovery of anti-Formin-like 1 protein (FMNL1) antibodies in membranous nephropathy and other glomerular diseases
- PMID: 35953506
- PMCID: PMC9372176
- DOI: 10.1038/s41598-022-17696-w
Discovery of anti-Formin-like 1 protein (FMNL1) antibodies in membranous nephropathy and other glomerular diseases
Abstract
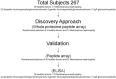
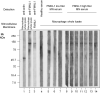
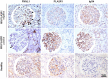
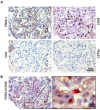
Evidence has shown that podocyte-directed autoantibodies can cause membranous nephropathy (MN). In the present work we investigated sera of MN patients using a high-density peptide array covering the whole coding sequences of the human genome encompassing 7,499,126 tiled peptides. A panel of 21 proteins reactive to MN sera were identified. We focused our attention on Formin-like 1 (FMNL1), a protein expressed by macrophages in MN patients tissues. High levels of anti-FMNL1 IgG4 were demonstrated in sera of MN patients with an orthogonal methodology (ELISA) contemporary demonstrating FMNL1 positive cells in kidney co-staining with CD68 in glomeruli. High levels of circulating anti-FMNL1 IgG4 were associated with lack of remission of proteinuria, potentially indicating that autoantibodies directed against cells other than podocytes, involved in tissue repair, might play a role in MN disease progression. High serum levels of anti-FMNL1 IgGs were also observed in other non-autoimmune glomerolonephrites, i.e. idiopathic and genetic FSGS, IgAGN. These findings are suggestive of a broader role of those autoantibodies in other glomerular disease conditions.
© 2022. The Author(s).
Conflict of interest statement
M.P., J.J.P, J.C.T, and K.C.L were Roche employees at the time of those researches. The other authors declare no competing interests.
Figures




Similar articles
-
Netrin G1 Is a Novel Target Antigen in Primary Membranous Nephropathy.J Am Soc Nephrol. 2022 Oct;33(10):1823-1831. doi: 10.1681/ASN.2022050608. Epub 2022 Aug 19. J Am Soc Nephrol. 2022. PMID: 35985817 Free PMC article.
-
Idiopathic membranous nephropathy: an autoimmune disease.Semin Nephrol. 2011 Jul;31(4):333-40. doi: 10.1016/j.semnephrol.2011.06.004. Semin Nephrol. 2011. PMID: 21839366 Free PMC article. Review.
-
Patients With Combined Membranous Nephropathy and Focal Segmental Glomerulosclerosis Have Comparable Clinical and Autoantibody Profiles With Primary Membranous Nephropathy: A Retrospective Observational Study.Medicine (Baltimore). 2016 May;95(21):e3786. doi: 10.1097/MD.0000000000003786. Medicine (Baltimore). 2016. PMID: 27227951 Free PMC article.
-
Prevalence, diagnostic value and clinical characteristics associated with the presence of circulating levels and renal deposits of antibodies against the M-type phospholipase A2 receptor in idiopathic membranous nephropathy.Nefrologia. 2014 May 21;34(3):353-9. doi: 10.3265/Nefrologia.pre2013.Dec.12291. Epub 2014 Feb 10. Nefrologia. 2014. PMID: 24798555 English, Spanish.
-
Is primary membranous nephropathy a complement mediated disease?Mol Immunol. 2020 Dec;128:195-204. doi: 10.1016/j.molimm.2020.10.017. Epub 2020 Nov 1. Mol Immunol. 2020. PMID: 33142137 Review.
Cited by
-
Membranous nephropathy: Clearer pathology and mechanisms identify potential strategies for treatment.Front Immunol. 2022 Nov 2;13:1036249. doi: 10.3389/fimmu.2022.1036249. eCollection 2022. Front Immunol. 2022. PMID: 36405681 Free PMC article. Review.
-
Mechanisms Limiting Renal Tissue Protection and Repair in Glomerulonephritis.Int J Mol Sci. 2023 May 5;24(9):8318. doi: 10.3390/ijms24098318. Int J Mol Sci. 2023. PMID: 37176025 Free PMC article. Review.
-
Technology Innovation for Discovering Renal Autoantibodies in Autoimmune Conditions.Int J Mol Sci. 2024 Nov 25;25(23):12659. doi: 10.3390/ijms252312659. Int J Mol Sci. 2024. PMID: 39684370 Free PMC article. Review.
-
Novel biomarkers and pathophysiology of membranous nephropathy: PLA2R and beyond.Clin Kidney J. 2023 Dec 11;17(1):sfad228. doi: 10.1093/ckj/sfad228. eCollection 2024 Jan. Clin Kidney J. 2023. PMID: 38213493 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

